ulsum D. Casey
Medical Morbidity and Mortality Following Revision Total Hip and Knee Arthroplasty
PREVENTING AND MANAGING ADVERSE CARDIAC EVENTS
GASTROINTESTINAL COMPLICATIONS
POSTOPERATIVE NEUROLOGICAL COMPLICATIONS AND NEUROLOGICAL DISEASES
MORTALITY FOLLOWING REVISION TOTAL HIP AND KNEE ARTHROPLASTY
INTRODUCTION
The perioperative management of medical conditions is an important aspect in the prevention of postoperative medical morbidity, especially in older patients undergoing extensive revision joint arthroplasty procedure. The following chapter identifies diseases and organ-specific complications and provides recommendations for perioperative management of these conditions.
PREVENTING AND MANAGING ADVERSE CARDIAC EVENTS
Preoperative Evaluation for Perioperative Myocardial Ischemia Risk Formal preoperative assessment by a medical physician or anesthesiologist of a patient’s cardiac status is a necessary step in preparation for surgery. Anesthesia has a large impact on cardiovascular physiology, and unidentified or untreated critical myocardial ischemia can lead to an adverse myocardial event in the intraoperative or immediate postoperative period. As opposed to respiratory and neurologic complications, the type of anesthesia (general or spinal) has no effect on rates of postoperative adverse myocardial events.
Orthopaedic surgeries are considered intermediate-risk procedures. Intermediate risk implies a <5% chance of a postoperative adverse cardiac event of death. The amount of blood loss, fluid shifts, and length of anesthesia are three of the main components of a surgery that contribute to this risk. Patient-specific risk factors include history of myocardial infarction, unstable angina, diabetes, renal insufficiency, advanced age, congestive heart failure, peripheral vascular disease, and poor functional status. Patients with known coronary artery disease whose ischemic threshold is <60% of age-predicted maximal heart rate during a noninvasive cardiac stress test are the patients at highest risk (odds ratio 7.002; 95% CI 2.79 to 17.61).1 Aortic stenosis is an important, underestimated risk factor for perioperative complications. Patients with severe aortic stenosis have a significantly higher postoperative rate of myocardial infarction and death as compared to those with moderate and no aortic stenosis (31%, 11% and 2%, respectively).2
Patients requiring lower extremity revision often have restricted mobility due to pain. As a result, assessing cardiovascular functional status may be difficult if they are unable to achieve four to ten metabolic equivalents of activity (i.e., climb one flight of stairs nonstop without limiting chest discomfort or dyspnea) due to joint pain. If the patient has no obvious recent myocardial ischemia, but functional status cannot be assessed, then noninvasive, pharmacological myocardial stress testing to rule out significant coronary artery disease is indicated.
Rarely is preoperative cardiac intervention necessary for the sole purpose of reducing risk of an adverse postoperative cardiac event. In the vast majority of cases, optimizing medical Introduction management of stable coronary artery disease with beta-blocker therapy is sufficient to reduce risk to that of the general population. If beta-blockade is contraindicated, diltiazem may be a suitable alternative; however, this has not been evaluated in a prospective, randomized controlled fashion. Specific cardiac interventional procedures and surgery are to be done preoperatively only if the intervention is indicated, irrespective of the current situation. In the case of elective revisions, it is ideal to wait at least 6 weeks following interventional cardiac procedures in order to allow stents to endothelialize and the course of antiplatelet agents to be completed. “Special and rapidly evolving recommendations apply to patients with drug eluting stents and these should be followed according to latest guidelines.”
Cardiac Arrhythmias For patients with preexisting cardiac arrhythmias, medical management is the same as in the nonoperative setting. Therapy is directed at correcting the underlying causes and then at the rhythm disturbance itself. Implantable pacemakers or intracardiac defibrillators should be turned off immediately before surgery and restarted postoperatively.
Postoperative arrhythmias are common complications and occur most often in patients with structural heart disease. Other than sinus tachycardia, atrial fibrillation is the most common postoperative arrhythmia. Precipitating factors for atrial tachyarrhythmias include such transient occurrences as hypoxemia, electrolyte abnormalities, catecholamine surge, or myocardial ischemia of volume overload. Medical or cardiology consultation should be obtained. If the patient is experiencing hemodynamic compromise or loses consciousness due to a tachyarrhythmia other than sinus tachycardia, cardiac monitoring and prompt electrical cardioversion are indicated. The most important aspects of immediate care include ensuring that heart rate is controlled and systolic blood pressure maintained. Treat a rapid ventricular response in atrial tachyarrhythmias with beta-blockers or calcium channel blockers that have effect on the AV node. If the atrial arrhythmia immediately subsides, outpatient evaluation may be the most appropriate avenue for evaluation and would be at the discretion of the cardiologist, hospitalist, or other medicine physician performing the consultation. If, however, the tachyarrhythmia is sustained, echocardiographic evaluation for structural cardiac abnormalities (such as left atrial enlargement) is indicated. In patients with atrial fibrillation, anticoagulation to prevent a cerebrovascular event may be indicated and will have to be weighed in concert with perioperative bleeding risk.
Congestive Heart Failure As the population ages, congestive heart failure (CHF) is one of the most common chronic medical conditions in the general population today.
Patients with chronic heart failure are at significant risk for an episode of acute decompensation during the immediate postoperative period. The changes in vascular tone and resultant fluid shifts associated with anesthetic alone are cause enough for an acute exacerbation of CHF. In this case, transient pulmonary edema in the setting of known ventricular dysfunction is most likely to occur between 48 and 72 hours following surgery with remobilization of extravascular fluids. Likewise, because of their known left ventricular dysfunction, these patients are much more vulnerable to being tipped “off balance” with transient episodes of ischemia, tachycardia, hypoxia, or mild to moderate infection of the urinary tract or lungs.
Pulmonary or peripheral edema postoperatively in patients with known CHF does not occur solely secondary to volume overload. Those who develop an acute worsening of their symptoms postoperatively should have a screening evaluation for other precipitating factors for CHF exacerbations such as myocardial infarction, infection, and tachyarrhythmias. Any underlying cause should be treated according to standard evidence-based care in the nonoperative setting. Only if no precipitating cause is found, should the cause be thought to be secondary to postanesthetic fluid shifts and resultant volume overload. Paying inadequate attention to these symptoms is dangerous. Among patients with coronary artery disease undergoing noncardiac surgery, those with concomitant chronic CHF suffer more mortality and have a significantly higher 30-day readmission rate following their surgery.3
The goal of therapy of CHF is resolution of symptoms and of the hemodynamic derangement leading to the decompensation. Treatment associated with either an exacerbation of CHF or a new diagnosis is the same. Patients need diuresis, optimization of their electrolytes, and correction of significant anemia to maximize oxygen-carrying capacity. In the presence of hypoperfusion or renal dysfunction, ionotropic agents including digoxin, and vasodilators should be used concomitantly with diuretic therapy. Consideration should be given during the acute episode to address long-term, chronic therapy which is dependent on the physiology of heart failure and effective cardiac output. Typically, regimens include a combination of agents including diuretic therapy, an angiotensin-converting enzyme (ACE) inhibitor (or other vasodilator if ACE inhibitors cannot be used), possibly digoxin, and often a beta-blocker. To ensure that patients are on optimal therapy following a new diagnosis or acute exacerbation of chronic failure, a cardiology consultation should be obtained to review and prescribe appropriate medical regimen.
Myocardial Ischemia and Infarction The optimal method for diagnosing perioperative myocardial infarction (MI) is poorly studied; therefore, no single strategy can be recommended. The constellation of indicators includes clinical symptoms, intraoperative or postoperative ECG changes, and elevation of the CK-MB or troponin. Many patients will not have chest pain because of postoperative narcotic pain control; hence, the diagnosis is challenging. Routine surveillance for a perioperative MI should be reserved for patients who developed signs of cardiac ischemia or hemodynamic compromise intraoperatively, or for those who are considered high risk by patient-specific or surgery-specific risk factors. In these cases, it is reasonable to obtain an ECG preoperatively, immediately postoperatively, and every day for 2 days.4 Cardiac enzymes should be drawn postoperatively to rule out MI when there are signs and symptoms of myocardial ischemia or significant changes in hemodynamics. Routine cardiac telemetry monitoring in the absence of documented myocardial ischemia or hemodynamic compromise is controversial and of low yield.
Role of Pain Management in Postoperative Myocardial Ischemia Effective pain management, with epidural and patient-controlled analgesia, leads to a reduction in postoperative catecholamine surges and hypercoagulability. Appropriate use of epidural analgesia has repeatedly been shown to decrease postoperative myocardial infarctions.5–7
Role of Anemia in Postoperative Myocardial Ischemia There is a paucity of data regarding the role of anemia in postoperative myocardial ischemia. Theoretically, a reduction of oxygen-carrying capacity in conjunction with the increase in myocardial workload during the perioperative period would increase the risk of suffering a significant adverse cardiac event. There is a suggestion by one study that patients with a hematocrit <28% had a significantly higher incidence of MIs as compared to those whose hematocrit was >28% (10 out of 13 vs. 2 out of 14 with MIs, respectively).8
Treatment of Postoperative Myocardial Ischemia
Despite even optimal perioperative management, some patients will have perioperative MI, which is associated with a 40% to 70% mortality rate.9 In the setting of an evolving postoperative myocardial ischemic event, aspirin and beta-blocker therapy should be given immediately. The use of other anticoagulants and antiplatelet therapy carries obvious bleeding risk after a major revision surgery. Treatment decisions must be made weighing the risks and benefits of blood loss or hematoma versus the risks of incompletely treated myocardial ischemia. The site of the surgical procedure and duration of time since surgery are the determining factors. In the setting of a symptomatic ST segment elevation MI, coronary angiography with angioplasty should be strongly considered.10
ADVERSE PULMONARY EVENTS
Pulmonary complications significantly contribute to postoperative morbidity and mortality. It has been estimated that almost 25% of deaths occurring within 6 days of surgery are related to postoperative pulmonary complications.11 The definition of what constitutes a postoperative pulmonary complication varies greatly. Atelectasis, pneumonia, acute respiratory distress syndrome, and respiratory failure have all been classified as postoperative pulmonary complications. For the purposes of this chapter, only postoperative respiratory failure and pneumonia will be discussed in detail.
Respiratory Failure Postoperative respiratory failure is obviously the most serious postoperative pulmonary complication. It is defined as mechanical ventilation for more than 48 hours after surgery or reintubation and mechanical ventilation after postoperative extubation. The inpatient mortality rate is reported to be as high as 40% to 42%.12
Postoperative Pneumonia Nosocomial pneumonias in surgical patients are characterized by a high frequency of early-onset infections, a high proportion of Gram-negative and staphylococci bacteria isolates, and a low mortality rate. The majority of cases are diagnosed between the fourth and fifth day postoperatively but may occur up to 9 days postoperatively. Initial antibimicrobial therapy of postoperative pneumonia should empirically cover Enterobacteriaceae, streptococci and Staphylococcus aureus.13 Antibiotics should be tailored to local microbial resistance patterns and bacterial sensitivities once reported.
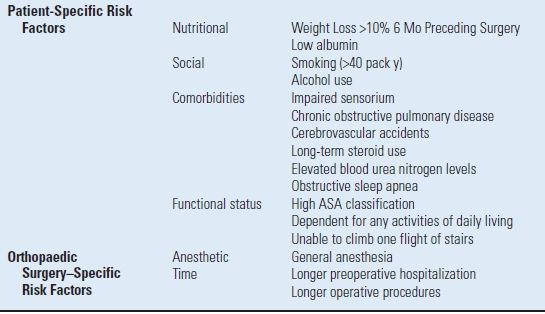
Risk Factors for Postoperative Respiratory Failure and Pneumonia The acquisition of pneumonia or respiratory failure postoperatively is associated with patient-specific preoperative markers and surgery-specific factors11,12,14–17 (Table 3.1).
TABLE 3.1 Adverse Pulmonary Events
Perioperative Measures to Reduce Risk of Postoperative Pulmonary Complications Preoperative Patients with COPD should be aggressively treated to optimize exercise capacity, symptoms, and airflow obstruction on physical examination. Elective surgery should be deferred if an acute exacerbation is present. The treatments are the same as those for patients who are not about to have an operation. Combinations of bronchodilators, physical therapy, antibiotics, smoking cessation, and corticosteroids reduce the risk of postoperative pulmonary complications.18–20 Routine preoperative pulmonary function testing is no better than clinical findings and physical exam in predicting the risk of pulmonary complications postoperatively. There is no study that has demonstrated a pulmonary function threshold after which risk of pulmonary complication would prohibit surgical intervention (elective surgery included).20 Preoperative smoking cessation should be strongly encouraged.
Intraoperative and Postoperative Anesthesia and Analgesia Use of intraoperative regional anesthesia, rather than general anesthesia, can significantly reduce risk of postoperative pneumonia. Risk of pneumonia may also be decreased with the use of postoperative epidural opioid analgesia as compared to systemic opioid analgesia. This has not been specifically studied in orthopaedic patients.
Postoperative Chest physiotherapy to increase lung volume has been shown to decrease postoperative adverse pulmonary complications. Mechanisms to increase lung volume include deep breathing exercises, incentive spirometry, and intermittent positive pressure breathing. Every patient postoperatively should be instructed on deep breathing exercises and/or incentive spirometry.
GASTROINTESTINAL COMPLICATIONS
Postoperative Nausea and Vomiting With modern anesthetic procedures, the overall incidence of postoperative nausea and vomiting (PONV) is approximately 40%.21 It is important to avoid this problem if possible. It may begin immediately upon awaking from anesthesia and last up to 48 hours, and can significantly contribute to patients’ overall surgical experience by delaying discharge, increasing resource utilization, and decreasing patient satisfaction.22
Patient-specific risk factors for PONV include female gender, history of PONV or motion sickness, nonsmoking status, and postoperative opioid use. If a patient has one or two of these risk factors, then use of dexamethasone, scolopolamine, or serotonin antagonist is recommended. For patients with three or four of these risk factors, use dexamethasone plus a serotonin antagonist to help control their symptoms.
Postoperative Ileus Ileus refers to the general decrease in bowel contractility. With anesthetic, the bowel appropriately relaxes and ceases its normal function for a limited period of time. Within 3 to 5 days, the temporary ileus typically resolves as patients begin to pass flatus and resume normal bowel function. Patients whose bowel function does not return during the expected time frame could have either a mechanical small bowel obstruction (most typically following abdominal surgery) or, more likely in all other nonabdominal procedures, a prolonged ileus.
Clinically, patients will develop abdominal distension, obstipation, nausea, and vomiting. They will have varying amounts of generalized discomfort or pain depending on the etiology of the ileus, experiencing more pain if it is a mechanical obstruction. Physical exam will reveal the distension with decreased or absent bowel sounds. In the case of postoperative ileus, x-rays of the abdomen reveal diffuse bowel dilatation. Air-fluid levels are more commonly seen if the patient is experiencing a mechanical obstruction.
Postoperative ileus has many causes; so, a multimodality approach to treatment is the most logical. Cisapride, nonsteroidal anti-inflammatory drugs (NSAIDs), early enteral feedings, oral magnesium, and early walking are all portions of one program.23
Acute Colonic Pseudo-Obstruction Acute colonic pseudo-obstruction (ACPO) is characterized by massive colonic dilation in the absence of mechanical obstruction; synonyms include acute colonic ileus and Ogilvie syndrome.24,25 Ischemia and perforation are the feared complications of ACPO; spontaneous perforation has been reported in 3% to 15% of patients with a mortality rate of 50% or higher.26 The rate of perforation and/or ischemia rapidly increases with cecal diameters >10 to 12 cm and when the duration of distension exceeds 6 days.
ACPO presents with features of large bowel obstruction. ACPO should initially be treated conservatively (fasting with nasogastric decompression, cessation of narcotic and antiemetic therapy). Patients should be mobilized, if possible, and therapy for all possible contributing factors (electrolyte replacement, etc.) should be initiated. A rectal tube may need to be placed for decompression. Early on, serial abdominal exams are a must along with repeated abdominal x-rays in order to assess cecal diameter. Success rates with the use of these conservative measures for 3 to 6 days vary from 20% to 92%.27
Active intervention is indicated for patients deteriorating during initial conservative management with signs or symptoms of ischemia, perforation, significant pain, fever, leukocytosis, or respiratory compromise. Neostigmine is an anticholinesterase parasympathomimetic agent used for postoperative reversal of nondepolarizing neuromuscular blockade and in the treatment of myasthenia gravis and postoperative urinary retention.28 Given the parasympathetic stimulation from neostigmine, there is a significant risk of adverse cardiac events (bradycardia, asystole, hypotension). As a result, neostigmine must be administered in a cardiac monitored setting. If neostigmine is not successful, then mechanical decompression with colonoscopy may be necessary. If pharmacologic and endoscopic decompression fails, surgical management with cecostomy, colectomy, or percutaneous cecostomy should be considered. This surgical approach should be used as first-line treatment if there is evidence of overt perforation or signs of peritonitis.
From the practical viewpoint, patients with prolonged gastrointestinal problems after major hip or knee surgery are at risk for overanticoagulation or underanticoagulation if Coumadin is used as the antithromboembolic agent. In circumstances where oral intake is compromised for a prolonged period of time after operation, often injectable antithrombotic agents, such as low molecular weight heparin, are preferred.
RENAL COMPLICATIONS
The incidence of postoperative acute renal failure (ARF) ranges from 1.1% to 17%.29 Patients undergoing hip and knee procedures are at risk for ARF due to blood loss, volume depletion, intraoperative anesthesia, mechanical ventilation, fluid shifts, and sepsis.
Preventing the Development of Acute Renal Failure After Hip or Knee Surgery Identifying risk factors (age, history of renal or ventricular dysfunction) and perioperative management of hemodynamics, oxygenation, and nephrotoxic agents is important in preventing postoperative ARF. In general, patients fall into one of three categories: patients with known chronic kidney disease, patients with normal renal function, and patients at high risk for renal dysfunction. The last group tends to be patients with long-standing hypertension and/or diabetes who have pathological renal disease that is not clinically evident. This group of patients has a notable risk of developing ARF because small variations in fluid or use of nephrotoxic agents may precipitate renal failure.
General Strategies in Preventing Acute Renal Failure Postoperatively
- Adequate hydration and prevention of hypovolemia: Avoiding dehydration, hypovolemia and hypotension, decreased renal blood flow. By restoring adequate renal perfusion through volume expansion, most incidents of prerenal failure and acute tubular necrosis (ATN) can be prevented. The most effective intravascular volume repletion is achieved with packed red blood cells. Isotonic crystalloids including 0.9% sodium chloride, lactated Ringer, and isotonic sodium bicarbonate solutions will also remain intravascular and increase renal perfusion more than hypotonic solutions. Colloid-containing fluids may be used in certain circumstances such as liver failure or patients with nephrotic syndrome. Intravenous fluid solutions containing water and glucose will do little or nothing to increase end organ perfusion.
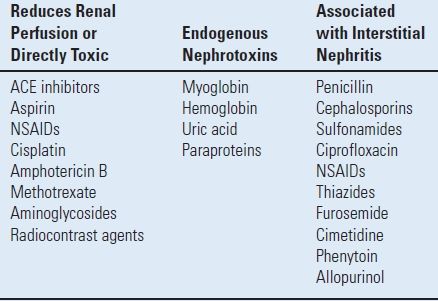
- Avoidance of nephrotoxic agents: Many medications/toxins may precipitate renal failure (Table 3.2). Although, most of these medications can be continued safely in healthy patients without the development of postoperative renal dysfunction, monitoring of urine output, blood urea nitrogen, and creatinine levels is advised. The use of NSAIDs perioperatively only transiently affects kidney function in healthy individuals. Therefore, NSAIDs need not be held in healthy patients perioperatively.30 However, they should be held if excessive bleeding is anticipated, especially in patients with known renal disease.
TABLE 3.2 Drugs and Toxins Associated with Renal Failure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








