Managing Bone Loss with Bulk Allograft
Gerard A. Engh
INDICATIONS
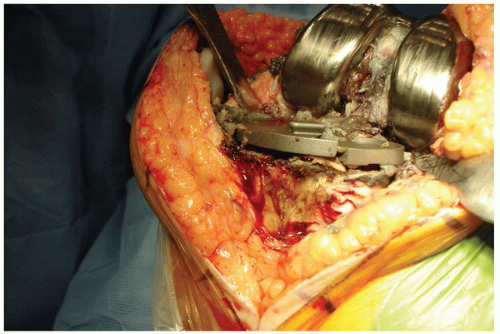
Bone loss is a complication encountered quite frequently at the time of revision total knee arthroplasty (TKA). The bone loss can be caused by progressive bone destruction secondary to debrisgenerated osteolysis or can be caused by implant loosening, breakage, and migration that occurred over time (Fig. 20-1). Also, bone loss can result from multiple revision procedures in which additional bone was removed with each surgery to obtain a satisfactory fixation interface for new components.
As a rule, implant augments can be used in the femur or tibia to manage defects that extend 10 to 15 mm into the metaphyseal bone. If the depth of the bone loss in the metaphyseal segment is greater than 15 mm, the only reasonable options for repairing such a defect would be to replace the lost bone with a large structural allograft (femoral head), impaction allografting, or use of a trabecular metal cone (an augment that is not integral to the component itself). In such cases, restoration/reconstruction of bone stock is beneficial to achieve satisfactory fixation for the revision component.
PREOPERATIVE CLASSIFICATION OF BONE DEFICIENCY
A useful tool in the planning for revision surgery is classifying the bone defect from preoperative radiographs (Fig. 20-2). According to the Anderson Orthopedic Research Institute (AORI) Bone Defect Classification (1), a type 3 defect has bone too deficient to support an implant. Type 3 bone defects are best managed with large structural allografts. Structural allografts provide additional support for the implant and an interface that rapidly heals to host bone. Impaction grafting also works relatively well to manage type 3 defects if the bone defect is contained in the metaphyseal region such that adequate cortical bone support is available to avoid implant subsidence or if wire mesh is added to provide additional support for an uncontained tibial defect (2,3).
CONTRAINDICATIONS
The contraindications for structural allografts in revision surgery are active infection, and in rare instances, severe bone loss such that the metaphyseal region is not adequate to contain a femoral head allograft. In other words, a structural femoral head allograft would be contraindicated in a knee with an absent metaphyseal segment. The more appropriate alternative for this type of extensive bone defect is either the combination of a segmental allograft and a long-stemmed revision component or a tumor prosthesis.
It is important to bypass an area of repaired bone damage to offload stress from an allograft. A diaphyseal filling stem provides long-term component stability and protects an allograft from stress overload. On rare occasions, a diaphyseal deformity may prohibit the use of a long-stemmed component.
PREOPERATIVE PREPARATION
An allograft bone segment can be obtained from a variety of sources. The American Society of Tissue Banks provides guidelines and requirements to ensure the safety of allograft bone. Such bone is readily available from the American Red Cross or other reputable tissue banks. Allograft bone must be maintained in a bone freezer at −70°C or delivered on the day before surgery and kept frozen with dry ice. The most viable source of large structural allografts is femoral heads retrieved at the time of primary total hip arthroplasty. The diameter of the bone segment is available with the packaged allograft. An allograft that is larger than the anticipated size of the bone defect is essential.
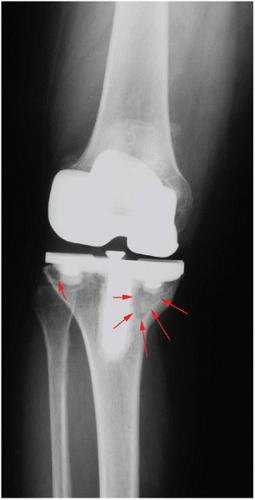 FIGURE 20-2 A large osteolytic lesion in the medial tibial plateau (arrows) is evident on this preoperative radiograph. A smaller lesion (arrow) is also noted in the lateral plateau. |
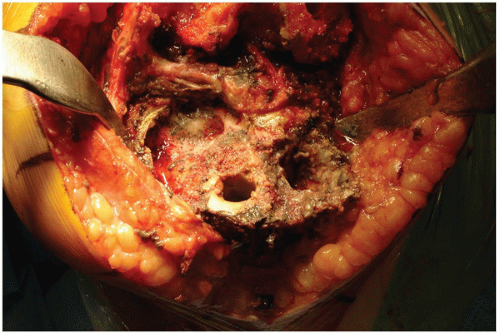 FIGURE 20-4 A broken titanium tibial tray that rested on an area of tibial osteolysis was removed and the defect classified (AORI type 3 tibial defect). |
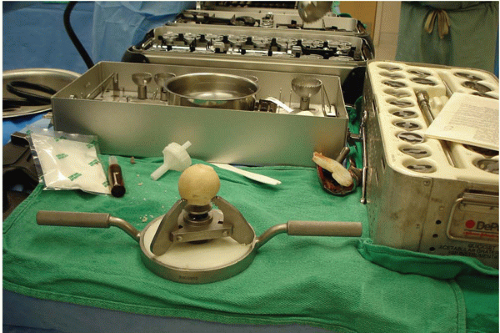
It is also essential to have the appropriate instrumentation for allograft preparation. The Allogrip (DePuy, Warsaw, IN) bone-holding device is best for preparing femoral head allografts (Fig. 20-3). The femoral head is anchored in the device for decortication and preparation. If the allograft is damaged or contaminated during preparation, it is important to have a back-up allograft available. The surgeon should always plan for the worst- case scenario to avoid the situation in which there is inadequate material to repair the bone deficiency.
ALLOGRAFTING TECHNIQUE
Preparation of Host Bone
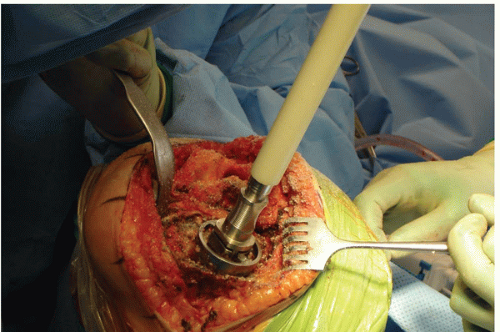
The surgical technique used to repair a major bone defect (AORI type 3 defect [1]) with an allograft begins with creating a host bed that will provide stability for the allograft and viability for rapid union between the allograft and the host bone. The base of the bone defect is generally filled with a combination of fibrous tissue, osteolytic membrane, and sclerotic underlying bone (Fig. 20-4). The membranous tissue must be vigorously débrided, and the sclerotic and dead cancellous bone is removed until encountering viable cancellous host bone at the base of the defect that can heal rapidly to an allograft. Often, a high-speed burr is needed to remove irregularly shaped sclerotic bone prior to using the hemispheric male-type reamer (Fig. 20-5) to create a hemispherical shape for the femoral head allograft. It is important to avoid creating an uncontained defect by removing additional cortical margins of the host bone during allograft preparation. However, it is necessary to ream deep enough such that the resulting bone defect is semicontained and viable bone is exposed for allograft union.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree