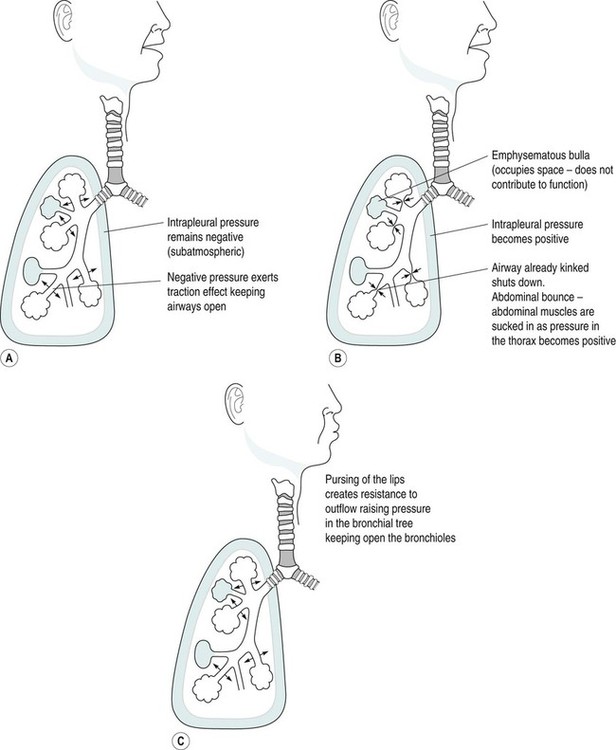
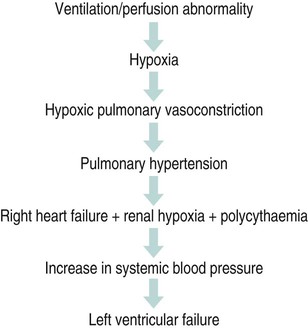
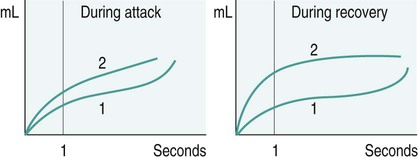
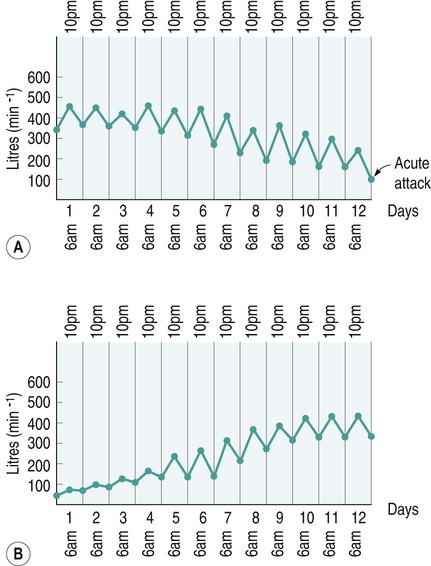
Diseases of the respiratory system are a major cause of illness worldwide and are increasingly important as a cause of mortality and morbidity (WHO 2004). In the UK they are the most common reason for consulting a general practitioner (GP) (Yelin et al. 2006) and a recent report on the ‘Burden of Lung Disease’ suggests that respiratory disease is second only to cardiovascular disease as a major cause of morbidity and mortality in the UK (BTS 2006a). Respiratory diseases encompass a range of diseases which affect the airways and other structures of the lungs, and although they are characterised by respiratory symptoms, it has become increasingly evident that chronic pulmonary diseases are associated with a range of non-pulmonary effects (Sinden and Stockley 2010; Decramer et al. 2012). In terms of the respiratory manifestations of respiratory disease, however, they can be divided broadly into obstructive and restrictive types – although most patients have elements of both. 1. Obstructive diseases include conditions in which there is a resistance to airflow, either through reversible factors such as bronchospasm or inflammation, or through irreversible factors such as airway fibrosis or loss of elastic recoil owing to damage to the airways and the alveoli. 2. Restrictive disorders are characterised by reduced lung compliance leading to the loss of lung volume, which may be caused by disease affecting the lungs, pleura, chest wall or neuromuscular mechanisms. These diseases are therefore different from the obstructive diseases in their pure form, although mixed restrictive and obstructive conditions can occur. Obstructive diseases are by far the most common and have major implications for patients and the economic burden on healthcare, as they require many years of medical intervention (Johnson et al. 2007). Therefore, their pathophysiology and treatment will be discussed initially in some detail. They are: Although historically COPD was considered as a disease of the lungs characterised by irreversible airflow obstruction, there is now increasing evidence that it presents with other associated comorbidities such as skeletal muscle dysfunction, cardiovascular disease, osteoporosis and diabetes (Sinden and Stockley 2010; Decramer et al. 2012). Therefore, because there is increasing recognition of the presence and impact of non-pulmonary manifestations, this has led to the now established international definition from the Global Initiative for Obstructive Lung Disease (GOLD). It is difficult to determine the exact prevalence of COPD in the community as the diagnosis of COPD is often only made following a first admission to hospital with an acute exacerbation of respiratory symptoms (Bastin et al. 2010). However, figures from general practice suggest that 5% of men and 2% of women will be diagnosed as suffering from COPD, although in the population as a whole it is estimated that 11% of men and 8% of women have evidence of obstructed airways when specifically tested by spirometry (BTS 2006a). Cigarette smoke is the main risk factor for COPD and is associated with 80–90% of cases (Ryter et al. 2010). However, not all smokers develop COPD and it is estimated that 15–20% of smokers will develop the pathology (Soares et al. 2010) with some evidence of women being more susceptible to the effects of cigarette smoke than men (Sørheim et al. 2010). However, it is also suggested that 10–20% of COPD cases are a result of occupational exposure to noxious particles, such as the combustion of solid fuels (Kurmi et al. 2010), and long-term exposure to traffic-related air pollution has been implicated in the development of COPD (Andersen et al. 2010). • stopping smoking and reducing exposure to atmospheric pollution; • maintaining physical fitness through participation in regular exercise; • maintenance of good general health, including good nutrition; As COPD is characterised by airways obstruction, the greater the obstruction, the lower the chance of survival (Pearson and Calverley 1995). Obstruction to flow is measured by forced expiratory volume in one second (FEV1) and together with age, FEV1 is the most important determinant of survival. Therefore, COPD can be classified according to the GOLD (2011) definition (Table 6.1). Table 6.1 Spirometric classification of patients with COPD according to GOLD (2011) FVC = forced vital capacity; FEV1 = forced expired volume in one second. Patients with COPD may also be classified according to the frequency of chest exacerbations each year (NICE 2010) or their level of breathlessness, as assessed by the Medical Research Council (MRC) dyspnoea scale (Table 6.2). Table 6.2 The Medical Research Council Dyspnoea Scale This is more common in middle-to-late adult life and in men more than women (Clarke 1991) although recent data suggest that mortality is now rising faster in women (Dransfield et al. 2006). Cigarette smoking is the chief culprit and, although in the UK over 20% of the adult population continue to smoke (DH 1997), only 15–20% of smokers may develop chronic bronchitis. The reason for this is probably genetic (Silverman et al. 2000) although the number of cigarettes smoked does have an effect on the progression of the disease. In the larger, more proximal airways, exposure to noxious particles stimulates mucus hypersecretion, with an increase in the size and number of goblet cells and submucosal glands. This results in a chronic cough with sputum production and is often termed chronic bronchitis (Jeffery 2000). There is also a decrease in the number and length of the cilia, all of which lead to retention of mucus, repeated infections, obstruction of airways and inflammation. In turn, this causes a release of toxins that further damage airway structure and function (Braman 2006). Atmospheric pollution (e.g. industrial smoke, smog and coal dust) will also predispose to the development of the disease, which is therefore more common in urban than in rural areas. It is more prevalent in socioeconomic groups 4 and 5 (Yelin et al. 2006) and is costly in terms of working days lost annually in the UK. The hallmark is hypertrophy, and an increase in number of mucous glands in the large bronchi and evidence of inflammatory changes in the small airways (Thurlbeck 1976). Some irritative substance stimulates over-activity of the mucus-secreting glands and the goblet cells in the bronchi and in the bronchioles, which causes secretion of excess mucus. This mucus coats the walls of the airways and tends to clog the bronchioles, which is functionally more important (West 2008). The cells increase in size and their ducts become dilated and may occupy as much as two-thirds of the wall thickness (West 2008). The airways become narrowed and show inflammatory changes, which results in mucosal oedema, thus further decreasing the diameter of the airways. The ciliary action is also inhibited. Airflow limitation in chronic bronchitis is more closely related to the dimensions of the distal (small) airways than proximal (large) airways (Hasegawa et al. 2006) and this narrowing of the lumen of the airways is further emphasised during expiration by the normal shortening and narrowing of the airways. Consequently, the airways obstruction is enhanced during expiration, with resulting trapping of air in the alveoli. The lungs gradually lose their elasticity as the disease progresses. They will gradually become distended permanently, which may eventually cause extensive rupture of the alveolar walls. After repeated exacerbations caused by infection there is widespread damage to the bronchioles and the alveoli with fibrosis and kinking occurring, as well as compensatory over-distension of the surviving alveoli. This is closely linked to and contributory to the development of emphysema (Hogg 2004). The prevalence of the condition is probably highest in England when compared with the rest of Europe, especially in the major centres of industry – although there is often a family history of the disease. It is more common in males (Corda et al. 2006). Congenital or primary emphysema may be caused by alpha1-antitrypsin deficiency. This is a rare inherited condition that affects 1 person in 4000 and results in the complete absence of one of the key anti-protease systems in the lung (Corda et al. 2006). The consequence is the early development of COPD, especially if the patient is already a smoker (Senn et al. 2005). Although alpha1-antitrypsin deficiency is responsible for less than 1% of cases of emphysema, its hereditary nature means that it is worth diagnosing. It should, therefore, be considered in any young COPD patient. Emphysema may be secondary to other factors, such as: • obstructive airways disease, e.g. asthma, cystic fibrosis, chronic bronchitis; • occupational lung diseases, e.g. pneumoconiosis; • compensatory to contraction of one section of the lung, e.g. fibrous collapse or removal when the remaining lung expands to fill the space. Emphysema is usually of the panacinar (panlobular) type. In centrilobular emphysema the upper zones of the lung are usually affected. This causes gross disturbance of the ventilation/perfusion relationship as there is a relatively well preserved blood supply to the alveoli but the amount of oxygen reaching the capillary is decreased owing to the damage to airways proximal to the alveoli. Panacinar emphysema predominantly affects the lower lobes; lower lobe involvement is more common in individuals with alpha1-antitrypsin deficiency (Stavngaard et al. 2006). This has a less drastic effect on the ventilation/perfusion relationship as the blood supply in the damaged areas is decreased in proportion to the decreased ventilation in those areas. Smoking causes the clustering of pulmonary alveolar macrophages (which are the major defence cells of the respiratory tract) around the terminal bronchioles. These macrophages are abnormal in smokers and release proteolytic enzymes that destroy lung tissue locally. Polymorphonuclear leucocytes, necessary to combat infection in the lung, release an enzyme that also destroys lung tissue. The defence mechanism against the unwanted action of these enzymes lies in the serum alpha1-antitrypsin, which is normally present in the airway lining fluids. Oxidants released by both cigarette smoke and the leucocytes tend to inactivate the anti-proteolytic action of the alpha1-antitrypsin, thus causing destruction of lung tissue as seen in centrilobular emphysema (Stavngaard et al. 2006). Subsequently, the walls of the airways become weak and inelastic owing to the damage caused by repeated infections. They tend to act as a one-way valve as the walls collapse on expiration. This causes air trapping and consequent increase in the intra-alveolar pressure during expiration. The alveolar septa break down and form bullae (Figure 6.1). This occurs in patients with COPD and, together with the energy-requiring consequences of chronic infection and inflammation, leads to increased work of breathing (Donahoe et al. 1989). The patient becomes progressively more short of breath as the disease progresses. Shortness of breath occurs initially on exertion, but as the disease progresses it will gradually occur after less and less activity and finally at rest. This disabling breathlessness is what prevents the patient from working and gradually transforms the patient’s state into one of severe exercise intolerance and disability (Folgering and von Herwaarden 1994). In a patient where an emphysematous component predominates, shortness of breath may progress more rapidly during the progression of the disease. In the patient where emphysema predominates they may present with a ‘fish-like’ inspiratory gasp, which is followed by prolonged, forced expiration usually against ‘pursed lips’. The latter creates back-pressure which tends to prevent airways shutdown during expiration. Owing to increased intrathoracic pressure the jugular veins fill on expiration. A ‘flick’ or ‘bounce’ of the abdominal muscles may be seen on expiration as the outward flow of air is suddenly checked by the obstruction of the airways (Figure 6.2). This may occur in the later stages of COPD. The impaired gas exchange in COPD caused by the disruption of ventilation and perfusion and the resulting hypoxia leads to widespread hypoxic pulmonary vasoconstriction. This leads to an increase in pulmonary vascular resistance resulting in pulmonary hypertension (Vender 1994). The increase in the pressure within the pulmonary artery will create a resistance, which the right ventricle must overcome. This eventually leads to hypertrophy and dilatation, a condition known as ‘cor pulmonale’. Right heart failure leads to an increased pressure in the peripheral tissues resulting in the development of peripheral oedema. The combination of renal hypoxia and the increase in blood viscosity from polycythaemia increases the systemic blood pressure and eventually leads to left heart failure. The development of pulmonary oedema, which exacerbates the hypoxia and low cardiac output in patients with COPD, leads to a terminal stage of the disease. The mechanism of this cycle is illustrated in Figure 6.3. There is reduction of FEV1 and the forced vital capacity (FVC) is grossly reduced. The residual volume (RV) will be increased at the expense of the vital capacity (VC) because of air trapping and the inability of the expiratory muscles to decrease the volume of the thoracic cavity. The expiratory flow–volume curve is grossly abnormal in severe disease; after a brief interval of moderately high flow, flow is strikingly reduced as the airways collapse and flow limitation by dynamic compression occurs. A scooped-out appearance is often seen (Decramer 1989). Ventilation/perfusion mismatch is inevitable in COPD and leads to a low arterial oxygen pressure (PaO2) with or without retention of carbon dioxide (CO2). As the disease becomes severe, the arterial carbon dioxide pressure (PaCO2) may rise, and there is some evidence that the sensitivity of the respiratory centre to CO2 is reduced (Fleetham et al. 1980), which may leave the respiratory stimulus dependent upon the hypoxic drive. However, more recent evidence suggests that the administration of high levels of oxygen (>70%) in patients with COPD may increase hypercapnia owing to the reversal of pre-existing regional pulmonary hypoxic vasoconstriction, resulting in greater dead space (Crossley et al. 1997). The cause of loss of skeletal muscle mass is not fully understood, but may be caused by a number of factors, including energy imbalance, systemic inflammation, disuse atrophy and hormonal changes (Loring et al. 2009). As a result of pulmonary impairments, patients with COPD often present with dyspnoea and hyperinflation, which results in increased work of breathing (Loring et al. 2009). As well as loss of muscle mass, skeletal muscle abnormalities have been found in COPD. These include morphological and metabolic abnormalities (Seymour et al. 2009). Bernard et al. (1998) found a 30% decrease in thigh cross-sectional area in patients with moderate-to-severe COPD compared with control subjects. There is also evidence of change in muscle fibre type from fatigue resistant fibres to those which have less oxidative enzyme activity and altered mitochondrial function. These are likely to contribute to an inefficient muscle metabolism and loss of muscle strength and endurance (Maltais et al. 1997). Cardiovascular disease is a common cause of mortality in patients with COPD, which accounts for approximately 30% of deaths in patients with COPD (McGarvey et al. 2007). This may be attributed partly to common risk factors, such as exposure to cigarette smoke and reduced physical activity. Nevertheless, studies have shown that after controlling for these factors, patients with COPD still have an elevated risk of cardiovascular disease (McGarvey et al. 2007). Loss of bone mineral density and osteoporosis is a significant problem in patients with COPD, affecting between 32 and 60% of patients (Bolton et al. 2004). Bone mineral density has been related to severity of airways obstruction in COPD (Bolton et al. 2004) and to exercise capacity in men with COPD. Within the spectrum of COPD, two extremes of clinical presentation are recognised: type A and type B. At one time these were classified either as ‘pink puffers’ (type A) or ‘blue bloaters’ (type B) to correlate with the relative amounts of emphysema and chronic bronchitis respectively (Figure 6.4). While these definitions are over-simplistic, it is worth remembering that patients can present in dramatically different ways (Kesten and Chapman 1993). Patients with this syndrome often show the following symptoms: • comparatively mild dyspnoea; • copious sputum which may become infected; • low PaO2 and high PaCO2 (PaO2 > 8kPa; PaCO2 > 6.5kPa) because they tend to hypoventilate; • central cyanosis with cor pulmonale; • an increased residual volume but normal total lung capacity, hence tidal volume is decreased. Patients with this syndrome often show the following symptoms: • little or no sputum production; • relatively normal PaO2 and PaCO2 (PaO2 < 8 kPa; PaCO2 normal/low) because of hyperventilation early on in the disease; • central cyanosis and the development of cor pulmonale in the later stages of the disease; • generally no peripheral oedema until the late stages of the disease; • an increased total lung capacity because of hyperventilation. 1. Decrease the bronchial irritation to a minimum. The patient should be advised to stop smoking and avoid dusty, smoky, damp or foggy atmospheres. Occupation or housing conditions may need to be changed. 2. Control infections. All infections should be treated promptly as each exacerbation will cause further damage to the airways. The patient should have a supply of antibiotics at home and receive a vaccination against influenza each winter. The main organisms of concern are Streptococcus pneumoniae and Haemophilus influenzae, which are usually sensitive to amoxycillin or trimethoprim. 3. Control bronchospasm. Although bronchospasm is not a prominent feature of this disease, drugs (e.g. salbutamol) may be given to relieve the airways obstruction as much as is possible. 4. Control/decrease the amount of sputum. Patients with chronic bronchitis may present with excessive bronchial secretions and are usually able to eliminate this by themselves. However, during an episode when secretions may become difficult to eliminate, physiotherapy techniques, including postural drainage; active cycle of breathing technique (ACBT), including Forced Expiratory Technique (FET), Positive Expiratory Pressure (PEP) and oscillating PEP; may aid expectoration (Bott et al. 2009). 5. Oxygen therapy. Oxygen must be prescribed and should be given with great care, especially if a normal pH indicates a chronic compensated respiratory acidosis (renal conservation of bicarbonate ions (HCO3) to maintain pH within 7.35 to 7.45). In this instance HCO3 is raised above 24 mmol/L whilst PaO2 is low and the PaCO2 is raised. Controlled oxygen may be given via a Ventimask (or equivalent) with careful monitoring of blood gas levels. 6. Long–term oxygen therapy (LTOT). As respiratory function deteriorates, the level of oxygen in the blood falls leading to an increase in pulmonary hypoxic vasoconstriction and deterioration in cardiac function. In 1981, the Medical Research Working Party examined the effects of supplementary low concentrations of oxygen (24%) for 15 hours a day in COPD and found that it reduced three-year mortality from 66% to 45%. The British Thoracic Society Guidelines (2006b) suggest that patients who have a PaO2 of less than 7.3kPa, with or without hypercapnia, and a FEV1 of less than 1.5 litres, should receive LTOT. This therapy should be considered also for patients with a PaO2 between 7.3 and 8.0kPa and evidence of pulmonary hypertension, peripheral oedema or nocturnal hypoxia. 7. Noninvasive ventilation (NIV). Indicated during an acute exacerbation with type II respiratory failure (see definition in section on Respiratory Failure) (Lightowler et al. 2003; Nava and Hill 2009). NIV offloads the diaphragm enabling it to rest whilst the exacerbation resolves (Fauroux et al. 2003). • The relievers are used to reduce bronchospasm and include the beta2 (β2) agonists (which may be short- or long-acting), the anticholinergics and the xanthene derivatives. • The preventers may be used to prevent bronchial hyper-reactivity and reduce bronchial mucosal inflammatory reactions – they include the corticosteroids. Anticholinergic bronchodilators work by preventing bronchoconstriction, mediated by the parasympathetic nervous system. Two agents are currently available: ipratropium bromide and oxitropium bromide. Most studies suggest that these agents are at least as potent as β-agonists when used alone in COPD (Tashkin et al. 1986). A short-acting bronchodilator (β2-agonist or anticholinergic) used ‘as required’ is recommended as initial therapy in the British Thoracic Society guidelines (BTS 2001). The precise mode of action of the xanthene derivatives such as theophylline and aminophylline remains somewhat uncertain, although they are moderately powerful bronchodilators. They have, however, been shown to improve symptoms in COPD by increasing the contractual ability of the diaphragm (Murciano et al. 1989). The role of inhaled steroids (beclomethasone, budesonide) in COPD will vary from patient to patient. Steroids work by reducing inflammation and reducing bronchial hyperactivity. Trials have shown that about 10–20% of COPD patients will improve significantly following a short course of high-dose oral steroids (Gross 1995). In acute exacerbations, when conventional inhalers have proved inadequate, nebulisers may be used to deliver a therapeutic dose of a drug as an aerosol within a fairly short period of time, usually 5–10 minutes (BTS 2006b). The type of nebuliser for home use consists of a compressor or pump, a chamber and a mask or mouthpiece. The compressor blows air into the chamber, where it is forced through a drug solution and past a series of baffles. The solution is converted into a fine mist, which is then inhaled by the patient through the mask or the mouthpiece. • to improve exercise tolerance and ensure a long-term commitment to exercise; • to give advice about self-management in activities of daily living; • to increase knowledge of the patient’s lung condition and control of the symptoms; • to relieve any bronchospasm, facilitate the removal of secretions and optimise gaseous exchange; • to improve the pattern of breathing, breathing control and the control of dyspnoea; • to teach local relaxation, improve posture and help allay fear and anxiety. This definition focusses on three important features of successful rehabilitation. 1. A multi-disciplinary approach, which may include respiratory physicians, physiotherapists, occupational therapists, dieticians, nurses, psychologists and therapy assistants. 2. Attention to physical and social function through exercise training, education, nutritional, psychological, social and behavioral interventions. There is now unequivocal evidence to suggest that pulmonary rehabilitation improves both exercise capacity and health-related quality of life (Lacasse et al. 1996; Guell et al. 2006). In essence, the components of a pulmonary rehabilitation programme include aerobic exercise training, education about the background of the disease, smoking cessation, compliance with medication, nutritional support and energy-conserving strategies for activities of daily living (ADLs). Pulmonary rehabilitation programmes may also include psychosocial support with regard to advice on benefits, sexual function and anxiety management. National Institute for Clinical Excellence (NICE) Guidelines (2010) recommend that all suitable patients with COPD should be offered pulmonary rehabilitation, even after an acute exacerbation. The potential for fatigue of the ventilatory muscles is now recognised as an important component of ventilatory limitation in patients with COPD (Moxham 1990; Green and Moxham 1993). Fatigue may be caused by a combination of: • increased mechanical load on the respiratory muscles; • reduced energy supply to the respiratory muscles (Roussos and Zakynthinos 1996). It has also been established that respiratory muscle weakness, which may be a predisposition to muscle fatigue, is present in patients with COPD (Clanton and Diaz 1995; Polkey et al. 1995). It therefore follows that training techniques, which might specifically target the respiratory muscles, may prove beneficial in improving exercise tolerance in patients with COPD who may develop respiratory muscle weakness because of a loss of muscle mass (Geddes et al. 2004). This is a cycle of breathing control, thoracic expansion exercises and the forced expiratory technique (FET), and has been shown to be effective in the clearance of bronchial secretions (Prior and Webber 1979; Wilson et al. 1995) and to improve lung function (Webber et al. 1986). This may also aid sputum removal and may be combined with the ACBT technique. The optimum position for effectiveness must be established with each individual, although postural drainage for the lower lobe segments may be difficult as some patients may not tolerate the head-down position or even lying flat. In many patients the ACBT alone may be effective for many in the seated position (Cecins et al. 1999) and changes in position should be used to optimise gaseous exchange. In the lateral position, the lower lung is always better ventilated, regardless of the side on which the subject is lying, although there still remains a bias in favour of the right side because of its larger size when compared with the left lung (Svanberg 1957). Perfusion is also preferential to the lower lung in the lateral position in the spontaneously breathing person (West 2008), although if pathology exists within the lowermost lung gaseous exchange may be compromised because of the presence of pulmonary hypoxic vasoconstriction, which cannot be overcome by gravity (Chang et al. 1993). Non-invasive positive-pressure ventilation (NIPPV) is therefore indicated for the delivery of intermittent positive pressure and may be applied via the nose or mouth using a silicone mask attached to a bedside ventilator. Unlike IPPV, NIPPV can be administered on a general ward for patients in respiratory failure (Sinuff et al. 2000). The ventilator is programmed to supplement the patient’s own respiratory effort and, if required, oxygen therapy may be given in conjunction with NIPPV. NIPPV can be used during an acute exacerbation and has been shown to improve quality of life and arterial blood gas pressures (Meecham-Jones et al. 1995) and to reduce mortality in patients with COPD (Brochard et al. 1995). Extrinsic (atopic) asthma occurs in younger age groups and is caused by identifiable trigger factors, such as specific allergens. Patients are usually sensitive to different factors (e.g. pollen, house dust mites, feathers, fur, dust, pollution and, occasionally, food, drugs and exercise) and have a family history of similar sensitivities. Atopic subjects show an immediate skin reaction, elicited by pricking the skin through a drop of antigenic extract. Exposure to the precipitating factor causes a mucosal inflammatory allergic reaction. This type of asthma tends to be episodic. House dust mites provide the most common positive skin test in Britain, being positive in 80% of children with severe asthma. Extrinsic asthma is common in young people and is associated with a family history of asthma, hay fever and eczema, although new evidence suggests that the presence of more than one of these factors is also associated with the development of asthma in later life (Porsbjerg et al. 2006). Intrinsic (non-atopic) asthma tends to occur in the older patient as a chronic condition. This type of asthma is precipitated by, or associated with, chronic bronchitis, strenuous exercise, stress or anxiety. Respiratory infections are also a common factor in precipitating acute attacks, although the majority of these are viral in origin and therefore may represent inappropriate use of antibiotics (Shiley et al. 2010). The condition can occur at any age but is most common in children, especially boys (ratio of about 3 : 2). Approximately 10% of children under 10 years of age in the UK have bouts of coughing and wheezing related to narrowing of the airways. Asthma accounts for more absences from school than any other chronic disease, although days lost from school may be under-estimated owing to the under-diagnosis and under-treatment of childhood asthma (Baena-Cagnani and Badelliono 2010). Childhood asthma generally remits after puberty but it may return in later life. Asthma that starts in middle age is more common in women than men and remission in this age group is rare. The majority of cases of asthma are mild, although the course of the disease is unpredictable. The mortality rate is unacceptably high and has shown a slow rise since the 1960s; in 2008 in England and Wales there were 1204 reported deaths as a result of asthma (BTS 2008). The rise in the prevalence of asthma has continued since 1988 but had been shown to decline by 2003 (Burr et al. 2006). However this decline in asthma prevalence is thought to be a result of better disease management, as more children are now using inhaled corticosteroids as a preventive treatment (Burr et al. 2006). However, despite the rise in the use of corticosteroids under-treatment and inadequate appreciation of the severity of asthma by patients and doctors are important factors in determining mortality, with up to 86% of asthma deaths being preventable. Those most at risk are the patients who under-estimate their symptoms. About 15–20% of asthmatics do not notice moderate changes in their airflow obstruction (National Asthma Campaign 2002) and may quickly deteriorate until they suddenly present with severe asthma. Patients with inadequately controlled, severe persistent asthma are at a particularly high risk of exacerbations, hospitalisation and death, and often have severely impaired quality of life (Peters et al. 2006). In all types of asthma an underlying problem seems to lie in abnormal reactivity of the airways; that is, they narrow excessively in response to stimuli which would not affect normal subjects (Bone 1996). The main pathological changes occurring during an asthmatic attack are: • spasm of the smooth muscle in the walls of the bronchi and bronchioles (bronchoconstriction); • oedema of the mucous membrane of the bronchi and bronchioles; At the initial stage of an attack the cough may be unproductive and ‘barking’ in nature. It causes an increase in bronchospasm and dyspnoea. As the attack subsides, the cough becomes productive of casts or plugs of sputum. Such plugs – made up of yellow viscid mucus and desquamated epithelial cells and eosinophils – are often very occasionally coughed up during acute attacks, which may produce a marked relief of symptoms. A cough may be the only presenting symptom of asthma, particularly in children (Corraco et al. 1979). This is rapid and there may be an increased drop in blood pressure during inspiration (>10 mmHg) owing to an exaggeration in intrathoracic pressure swings caused by severe airways obstruction (pulsus paradoxus). However, pulsus paradoxus may be absent even in very severe attacks of asthma. When it is present, the measurement is easily performed with a sphygmomanometer and provides a guide to progress and response to treatment (Pearson et al. 1993). Analysis of blood gases provides important information to help the management of severe asthma. The usual finding is of a low arterial PaO2 (hypoxaemia) caused by ventilation/perfusion mismatch and a low PaCO2 (hypocapnia) because of the effects of hyperventilation. Later in the disease process, the PaCO2 may be found to be high because the hyperventilation fails to compensate for the fact that there are many under-ventilated alveoli which are distal to the blocked bronchioles. When the PaCO2 is found to be increasing and the pH is low this should be a danger sign that the patient may be becoming tired and be likely to need assisted ventilation if immediate improvement cannot be achieved (BTS 2008). FEV1 and FVC drop during a severe attack with little sign of reversibility (Figure 6.5). However, if FEV1 is measured before and after giving bronchodilators, and there is a 15% increase in FEV1, this amounts to significant reversibility. The FEV1 may be less than 30% of FVC. Total lung capacity, FRC and RV may be increased because of over-inflation of the lungs. Recovery is associated with a reduction in these lung volumes. Recordings of the peak expiratory flow rate (PEFR) for a week at home will often make the diagnosis of asthma obvious (Prior and Cochrane 1980). PEFR dips in the morning, particularly during the recovery phase (Figure 6.6). If the dip is severe (less than 33% of predicted) then respiratory arrest may occur (BTS 2008). In a severe attack, the PEFR may drop below 100 L/minute. However, it should be noted that a normal PEFR when a patient is asymptomatic does not exclude a diagnosis of asthma (BTS 2008). As asthma is, by nature, a paroxysmal condition, acute attacks that are resistant to bronchodilators may occur. Such attacks are potentially life-threatening, so prompt and effective treatment is imperative. In general, clinical criteria are most helpful and the recognition by the person that his or her asthma symptoms are worsening. Table 6.3 lists warning signs of an acute severe attack. Table 6.3 Warning signs of an attack of acute severe asthma Unlike patients with long-standing COPD, patients with asthma may tolerate higher levels of oxygen to correct hypoxaemia. During an acute episode it is essential that oxygen therapy be titrated according to the level of PaO2. It may be evident on clinical examination, however, that the asthmatic patient also has evidence of COPD. In this situation examination of the blood gases will reveal whether the patient has had raised PaCO2 levels for some time (a chronic compensated respiratory acidosis will be evident on an arterial blood gas analysis) where the use of controlled oxygen will be required. If a patient is reaching a stage where the respiratory muscles are starting to fatigue the PaCO2 may rise. In this situation immediate medical attention is required as the asthma is deemed life-threatening and the patient is likely to require invasive mechanical ventilation (Richards et al. 1993). In a small proportion of asthmatic people, long-term oral corticosteriods (e.g. beclomethasone and budesonide) will be necessary. In circumstances when an attack supervenes very rapidly, a short course of oral steroids (prednisolone) is required. It cannot be emphasised enough that this approach is safe and certainly much safer than a poorly-controlled attack of asthma. There have been suggestions that the adverse effects associated with long-term steroids, such as osteoporosis, might be less common in asthma but this has been shown to be untrue (Adinoff and Hollester 1983). The introduction of inhaled corticosteroids in 1972 radically changed the management of asthma as side effects from oral steroids were prevented. β2 agonists, anticholinergic agents and corticosteroids are frequently prescribed in metered-dose inhalers (MDIs). The particles leaving an MDI do so with considerable velocity and even with a perfect technique of inhalation, only about 10% of the dose reaches the respiratory tract – the remainder is deposited in the mouth or swallowed (Davies 1973). The MDI does have the advantage of being small and portable and familiar to many asthmatics. The MDI can also be used with a spacer, which virtually removes oropharangeal deposition, thereby increasing lung deposition to 20–30% (O’Callahan and Barry 1997). Inhaled steroids can cause hoarseness and oral thrush so a spacer device may be prescribed to minimise deposition of large particles of the medication around the mouth and throat. Breath-activated devices (Figure 6.7) are primed before actuation and the MDI is triggered by inspiratory airflow. The airflow required is low and the triggering of the device quiet enough not to disturb the inspiration. β2 agonists, anticholinergic agents and corticosteroids can be prescribed in this form of inhaler.
Management of respiratory diseases
Introduction
Chronic obstructive pulmonary disease: basic issues
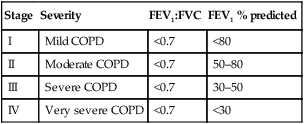
Stage
Severity
FEV1:FVC
FEV1 % predicted
I
Mild COPD
<0.7
<80
II
Moderate COPD
<0.7
50–80
III
Severe COPD
<0.7
30–50
IV
Very severe COPD
<0.7
<30
Grade
Degree of breathlessness related to activities
1
Not troubled by breathlessness except on strenuous exercise
2
Short of breath when hurrying on walking up a slight hill
3
Walks slower than contemporaries on the level because of breathlessness, or has to stop for breath when walking at own pace
4
Stops for breath after walking about 100 m or after a few minutes on the level
5
Too breathless to leave the house or breathless when dressing or undressing
Chronic bronchitis
Aetiology of chronic bronchitis
Pathology of chronic bronchitis
Emphysema
Causes and types of emphysema
Causes and predisposing factors
Types of emphysema
Pathology of emphysema
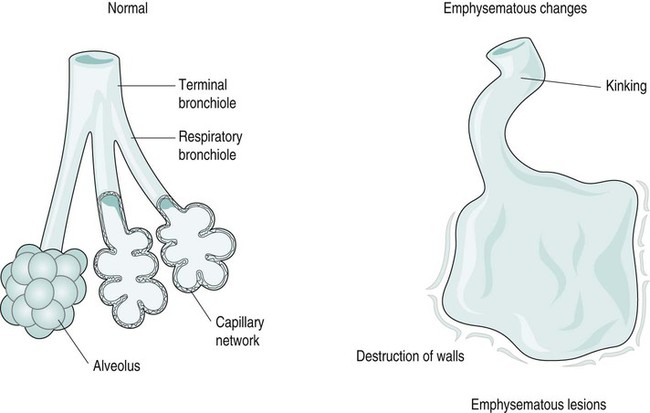
Clinical features of COPD
Dyspnoea or shortness of breath
Cor pulmonale
Lung function
Blood gases
Other non-respiratory manifestations of COPD
Loss of skeletal muscle mass
Reduced exercise tolerance
Increased cardiovascular risk
Osteoporosis
Varying clinical presentation in COPD
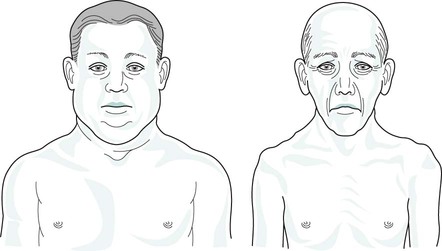
Blue bloaters
Pink puffers
Medical treatment of COPD
Medications
Anticholinergics
Xanthene derivatives
Corticosteroids
Drug delivery systems
Physiotherapy techniques in COPD
General aims of treatment
Treatment in the early stages
Increasing/maintaining exercise tolerance
Inspiratory muscle training
Removal of secretions
The active cycle of breathing technique (ACBT) (illustrated on Figure 9.9, p.178)
Postural drainage/positioning
Treatment in the later stages
Non-invasive positive-pressure ventilation
Asthma
Types of asthma
Extrinsic asthma
Intrinsic asthma
Aetiology and prevalence of asthma
Pathology of asthma
Clinical features of asthma
Extrinsic asthma
Cough
Pulse
Blood gases
Lung function
Acute severe asthma
Early warning signs
Signs of increasing severity
Increase in symptoms
Dyspnoea at rest
Sleep disturbance
Peak flow below 100 L/min
Increase in bronchodilator use
Deteriorating blood gases
Fall in peak flow
Pulsus paradoxus
Decrease in exercise tolerance
Tachycardia
Medical treatment of asthma
Oxygen therapy
Medications
Corticosteroids
Delivery of medication
Metered-dose inhalers
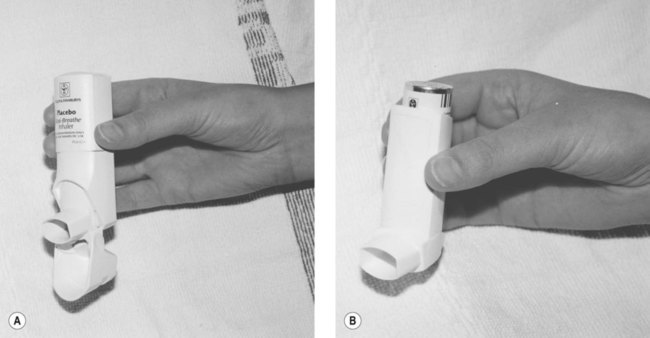
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Management of respiratory diseases