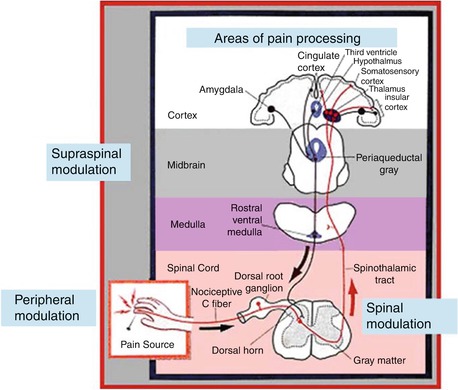
Fig. 36.1
Role of central hypersensitivity in the development of chronic pain (Adapted with permission from Curatolo et al. [11])
36.3 The Concept of Preemptive Analgesia and Multimodal Techniques
Preemptive analgesia involves the administration of analgesics before painful stimuli to prevent the establishment of central sensitization and thus the amplification of postoperative pain. It starts before surgery and covers both the period of surgery and the initial postoperative period. Preemptive analgesia prevents (or reduces) pathologic pain that is different from physiologic pain in several aspects: it is excessive (in intensity and spread) and can be activated by low-intensity stimuli (allodynia, hyperalgesia) and hyperpathia [15]. The interventions, therefore, must produce a dense blockade of appropriate duration to block the transmission of noxious afferent information from the peripheral nervous system to the spinal cord and the brain. In studies, the dose of morphine needed to prevent central hyperexcitability was one-tenth the dose when given prior to rather than after exposure to a brief noxious electrical stimulation [13]. Decreasing perioperative pain with preemptive techniques improves satisfaction, hastens discharge, spares opioid use (decrease constipation, sedation, nausea, and urinary retention), and may prevent the development of chronic pain.
Multimodal technique is a multidisciplinary approach to pain management with a goal to maximize the synergistic analgesic effect and minimize the side effects of the medications [16]. It takes advantage of the additive or synergistic effects of various analgesics, permitting the use of smaller doses with a concomitant reduction in side effects.
Preemptive analgesia means administration of analgesics prior to surgery in order to have central sensitization and amplification of pain after surgery.
36.4 Techniques of Pain Management
It is important to choose an effective analgesic regimen with minimal side effects to allow timely mobility and optimal functional recovery while decreasing postoperative morbidity and mortality. Although several treatment options involving various combinations of systemic analgesics and/or regional analgesia, with or without opioids, are available for postoperative pain, a gold standard has not been established. Recently, both individual and combined uses of a number of treatment options have been evaluated with respect to post-TKR acute pain control and include daily nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, opioid patient-controlled analgesia (PCA), epidural, oral opioids, periarticular local anesthetics, and peripheral nerve blocks [17–21].
36.5 Preoperative Patient Education
The preoperative patient education is one of the best techniques available to orient patients and their families because it provides information on what will happen to them throughout the whole process and substantially eases the “fear of the unknown.” This is a preemptive technique as the cerebral cortex response can be influenced by educating patients in the preoperative class about a successful pain-management program. Furthermore, the prevention of any onset of severe pain would prevent central sensitization of the cortex which magnifies pain, leading to its chronicity [16]. It should also be shared that pain after TKR persists in about one in eight patients despite an absence of clinical or radiological abnormalities [22].
36.6 Epidural Analgesia
Epidural medications may consist of a local anesthetic, an opioid, or a combination of both. A pure opioid epidural infusion may not provide adequate analgesia, and a pure local anesthetic may provide dense sensory and motor blockade, such that the patient may not be able to walk or void in the early postoperative period [23]. Thus, a combination creates a synergistic analgesic effect that allows lower concentration of each component in the solution. Continuous low-dose infusion has also been advocated as a method to control postoperative pain. Although they provide superior analgesia, they are also associated with technical failures, hypotension, ileus, urinary retention, motor block that limits ambulation, unrecognized compartment syndromes, and spinal hematoma secondary to anticoagulation [23]. Patient-controlled epidural analgesia (PCEA) offers higher analgesic efficacy and lower dose requirements than parenteral patient-controlled analgesia (IV PCA) and provides greater control and patient satisfaction than do either single-dose or continuous infusions. However, despite better pain control, patients still prefer IV PCA because of fewer technical problems, fewer side effects, and more uniform, sustained analgesia with more autonomy [5]. A Cochrane database review concluded that epidural analgesia may be useful after TKR for pain control, but the benefit must be weighed against the frequency of adverse effects [23].
36.7 Peripheral Nerve Blocks/Selective Sensory Blocks
The trend of early ambulation and discharge as well as same-day initiation of physical therapy has popularized the concept of peripheral nerve blocks. This trend has been aided by the technical advances in needles, catheters, nerve stimulation, and ultrasound detection of nerves. They minimize exposure to opioids and are ideally suited for patients sensitive to opioid-induced ileus, respiratory depression, and pruritus. Postoperatively, patients reported lower pain scores at rest and morphine consumption decreased for up to 8 h after transfer to the floor with placement of a femoral or femoral/sciatic block when compared to sham blocks [24]. Thus, advantages include effective postoperative analgesia, lower opioid consumption, improved rehabilitation, lower complications, and higher patient satisfaction [25]. Peripheral nerve block and catheter options include femoral with or without sciatic, lumbar plexus, adductor canal, and fascia-iliaca compartment blocks and have been discussed in detail elsewhere [19, 24, 25]. The role of selective sensory block for TKR is a relatively new concept under study [26].
36.8 Periarticular Injections
Periarticular injections and catheters have been shown to be safe and effective and have reduced the requirement of parenteral narcotics significantly compared to oral or intravenous opioids [16, 21]. The injections and catheters often use a combination of local anesthetics, steroid, epinephrine, NSAID, long-acting opioid, and antibiotic [16]. They are often used to compliment epidural and nerve blocks.
36.9 Opioids and Non-opioids Medications
When used preemptively as well as postoperatively, multiple opioid and non-opioid medications used in a multimodal way can significantly reduce postoperative pain scores, total opioid use and thus most of the adverse side effects, and length of hospital stay with patients having TKR. These include immediate- and controlled-release opioid formulations; tramadol; COX I, II, and III inhibitors; acetaminophen; steroids; anticonvulsants (gabapentin, pregabalin, carbamazepine); antidepressants (amitriptyline); NMDA antagonists (ketamine and dextromethorphan); and α2-agonists (clonidine, dexmedetomidine) and have been discussed in detail elsewhere [16–33].
36.10 Chronic Pain After TKR
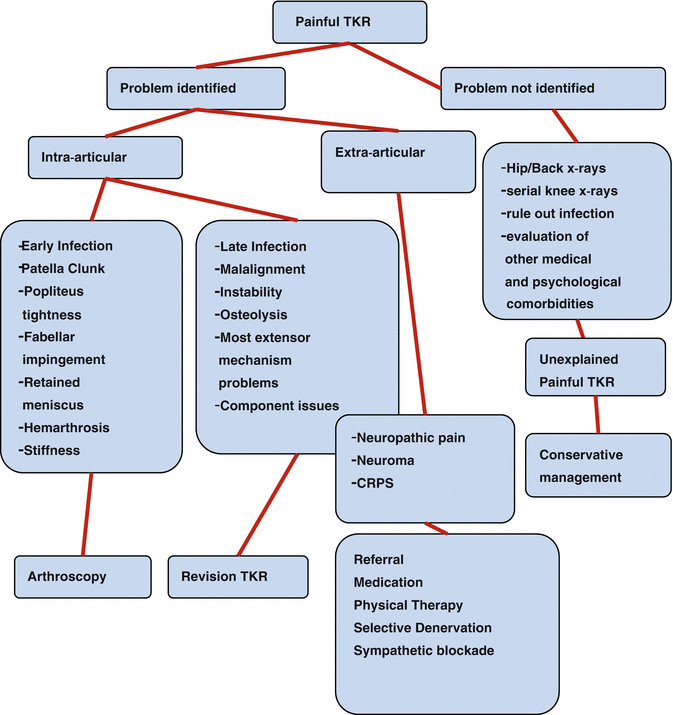
Some patients still have persistent chronic knee pain after a TKR, and their management can be difficult. The surgeon should be aware of both the common and rare underlying causes (Table 36.1) and should formulate a customized management algorithm [1–3] (Fig. 36.3). Depending on the etiology, management consists of three basic options: conservative approach, reoperations not needing an exchange of prosthetic components, and reoperations with exchange of at least one prosthetic component. For patients without any mechanical or infectious etiology, revision surgery often leads to suboptimal and unpredictable outcomes and should be avoided when possible [34, 35]. Mont et al. surgically explored the knees of 27 patients with debilitating pain after TKR without an identifiable cause; only 41 % resulted in an excellent or good result [35]. On the other hand, management of these patients conservatively has shown promising outcomes. In a prospective study of 116 patients, 15 had unexplained pain at the end of 1 year after TKR but were satisfied at the 5-year follow-up [22]. Another study of 622 TKRs found 24 to have unexplained pain, of which 55.5 % demonstrated improvement at the 5-year follow-up [36]. Moreover, in a randomized trial with 60 refractory painful TKRs, a single intra-articular botulinum toxin A injection provided significant short-term improvements in pain, global assessment scores, and function [37]. Thus, reassurance should be provided to the patient that improvement is possible after a conservative treatment. The details of surgical management have been discussed in detail elsewhere [1–3] and are beyond the scope of this chapter.
Intra-articular causes | Extra-articular causes |
|---|---|
Infection | Hip pathology |
Instability | Spine pathology |
Malalignment | Complex regional pain syndrome |
Aseptic prosthetic loosening | Vascular claudication |
Prosthetic failure/fracture | Neuroma |
Polyethylene wear and osteolysis | Bursitis |
Arthrofibrosis | Tendonitis |
Patellofemoral disorders: patellar maltracking, patellar baja, patellar clunk, unresurfaced patella, overstuffing, lateral patellar facet syndrome, etc. | Stress fractures |
Extensor mechanism problems: patellar fracture, patellar/quadriceps tendon rupture, etc. | Periprosthetic fractures |
Impingement: popliteus, component overhang, iliotibial band, fabella, retained cement, etc. | Medical and psychological comorbidities |
Miscellaneous: recurrent hemearthrosis, crystal arthropathy, heterotrophic ossification, tumors, metal/cement allergy, etc. | Miscellaneous: tumors, heterotrophic ossification |

Fig. 36.3
Management algorithm for painful total knee replacement
36.11 Neuropathic Pain
Neuropathic pain can be defined as pain caused by a lesion or dysfunction of the central or peripheral nervous systems. Neuropathic pain encompasses dysesthesia (abnormal sensation), allodynia (pain associated with normally non-noxious stimulus), hyperalgesia (increased response to a normally mildly noxious stimulus), and spontaneous pain. Treatment of neuropathic pain benefits from a multimodal approach because traditional opioid medications are not as effective – in fact, opioids should be used as second-line agents to treat neuropathic pain [38]. The various pharmacologic options to treat neuropathic pain have been shown in Table 36.2 [39]. As an adjuvant to neuropathic pain medication, alternative approaches such as topical capsaicin, transdermal lidocaine, ketamine, or fentanyl patches can help with poorly controlled neuropathic pain. Regular massage of the scar or the painful site may help and thereby prevent neglect of the joint and subsequent stiffness.
Table 36.2
Common medications for the treatment of neuropathic pain
Drug name | Medication mechanisms of action |
|---|---|
Tricyclic antidepressants (TCAs) | |
Amitriptyline | Inhibit the reuptake of norepinephrine |
Nortriptyline | |
Trimipramine | |
Selective norepinephrine reuptake inhibitors (SNRIs) | |
Duloxetine | Inhibit the reuptake of norepinephrine |
Venlafaxine | |
Anticonvulsants | |
Gabapentin | Binds to voltage-gated calcium channels |
Pregabalin | |
Topiramate | Blocks sodium channels, antagonizes glutamate receptors |
Carbamazepine | Stabilizes the inactivated stage of sodium channels |
Nontraditional opioids | |
Methadone | Opioid agonist, N-methyl-D-aspartate (NMDA) receptor antagonist, inhibits reuptake of norepinephrine |
Tapentadol | μ-Opioid agonist, inhibits reuptake of norepinephrine |
Tramadol | Weak μ-opioid agonist, inhibits reuptake of norepinephrine |
Miscellaneous | |
Capsaicin | Depletes and prevents accumulation of substance P in peripheral sensory neurons |
Ketamine | N-methyl-D-aspartate (NMDA) receptor antagonist; high doses stimulate μ- and Σ-receptors |
Lidocaine patch | Blocks initiation and conduction of nerve impulses by decreasing membrane permeability to Na+ |
36.12 Neuromas
When nerve ends are injured, occasionally ineffective and unregulated nerve regeneration occurs especially in close proximity to scars. When painful neuromas arise around the knee that are debilitating, a trial of conservative therapy is attempted for at least 6 months [40]. When conservative management (cortisone injections and topically delivered NSAIDs) fails, and surgery is considered to address the neuroma, patient selection is a priority in order to achieve good outcomes. Patients must respond favorably to a preoperative local anesthetic block or patch in order to predict outcome for surgical management of a neuroma [41]. Selective denervation is the procedure of choice for most surgeons, during which nerves supplying a neuroma are transected. Patient satisfaction rates reach up to 86 %, but patients need to be made aware of a 40 % minor complication rate including hypersensitivity from collateral sprouting of the normal, proximal stump due to nerve growth factor release [40, 41]. This is a self-limiting issue and responds well to desensitization therapy within 6 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree