CHAPTER 38 Management of draining wounds and fistulas
1. Identify medical conditions associated with fistula formation.
2. List three complications that contribute to mortality from fistulas.
3. Describe three ways to classify fistulas.
4. List the risk factors for postoperative fistula development and for irradiation-induced fistulas.
5. Identify the six objectives of medical management for the patient with a fistula.
6. List factors known to correlate with spontaneous closure and known to impede spontaneous closure of fistula tracts.
7. Describe surgical procedures commonly used to close or bypass fistula tracts.
8. List eight nursing management goals for the patient with a fistula.
9. Describe four essential assessments that guide the management of the patient with a fistula.
10. Explain the role of four different types of skin barriers and their indications for use.
11. Identify features to be considered when selecting a fistula pouching system.
12. Briefly describe the “bridging” technique and identify indications for its use.
13. Identify options for odor control in a wound managed with dressings and in a wound managed with pouching.
14. Describe the role of NPWT and closed wound suction in the management of an enterocutaneous fistula.
Epidemiology and etiology
Gastrointestinal fistulas are serious complications associated with high morbidity and high mortality (10%–20%), extended hospital stays, and increased costs (Kassis and Makary, 2008; Wong and Chang, 2008). Fistulas develop in a wide array of complex patient conditions and often are concentrated in large medical centers. Frequency data (i.e., incidence and prevalence) therefore tend to be skewed and difficult to interpret. Factors impacting on the prognosis of the patient with a fistula, however, are well known.
Medical conditions associated with fistula formation include technical difficulties with anastomosis, and coexisting disease such as inflammatory bowel disease, cancer, or diverticulitis. The following are risk factors that increase the likelihood of fistula formation when these medical conditions are present: severe malnutrition, sepsis, hypotension, vasopressor therapy, and steroid therapy. Enterocutaneous fistulas develop either spontaneously or postoperatively. In many reports, 75% to 85% of enterocutaneous fistulas are iatrogenic, occur postoperatively, and represent a leak in the anastomosis (Nussbaum and Fischer, 2006). Risk factors specific to developing a postoperative enterocutaneous fistula include reoperation requiring extensive lysis of adhesions, cancer, inflammatory bowel disease, emergency surgery where the bowel has not been adequately prepped, prior radiation therapy, and trauma surgery (Kassis and Makary, 2008; Nussbaum and Fischer, 2006). According to Wong et al (2004), postoperative fistulas caused by anastomotic breakdown are largely preventable by following basic surgical principles of anastomotic construction: (1) adequate blood supply, (2) lack of tension, and (3) good suture technique. Maykel and Fischer (2003) advocate specific prevention strategies when faced with emergency surgery: provide adequate intravenous fluids, ensure adequate circulatory support, keep the patient warm, and provide appropriately timed broad-spectrum antibiotics. In addition, when surgery is planned, they consider adequate nutritional preparation to be the most important step in preventing an anastomotic breakdown. The patients at highest risk for an anastomotic breakdown are the severely malnourished patients as manifested by a hydrated serum albumin level less than 3 g/dl and weight loss of 10% to 15% over a 4- to 6-month period (Kassis and Makary, 2008).
Approximately 25% of fistulas are acquired, that is, they develop spontaneously and are associated with an intrinsic intestinal disease (cancer, radiation, diverticulitis, inflammatory bowel disease, appendicitis) or external trauma. Spontaneous fistulas are generally resistant to spontaneous closure. Patients who have been treated for a pelvic malignancy are particularly vulnerable for fistula formation because of radiation damage to the rectum, anal canal, and gynecologic organs (Saclarides, 2002; Tran and Thorson, 2008). Irradiation triggers occlusive vasculitis, fibrosis, and impaired collagen synthesis, a process termed radiation-induced endarteritis. Because the endarteritis persists, complications may develop immediately after radiation or years later. Meissner (1999) reported that 17% of radiotherapy patients developed a fistula a mean of 3.4 years after receiving radiation therapy. Additional risk factors for irradiation-induced fistulas include coexisting processes such as atherosclerosis, hypertension, diabetes mellitus, advanced age, cigarette smoking, pelvic inflammatory disease, and previous pelvic surgery. Pelvic radiation doses exceeding 5000 cGy increase the incidence of bowel injury (Hollington et al, 2004; Saclarides, 2002; Tran and Thorson, 2008).
The three most common and highly significant complications associated with fistulas are sepsis, malnutrition, and fluid and electrolyte imbalance (Berry and Fischer, 1996). The loss of hypertonic protein-rich fistula effluent contributes to fluid and electrolyte depletion and malnutrition because of the loss of sodium bicarbonate and amino acids (Nussbaum and Fischer, 2006). Sepsis occurs as a result of abscess formation and compromised immune response due to poor nutritional status (Makhdoom et al, 2000). Uncontrolled sepsis and sepsis-associated malnutrition are primarily responsible for the mortality in patients with enterocutaneous fistulas, with mortality rates ranging from 6% to 20% (Hollington et al, 2004; Maykel and Fischer, 2003; Nussbaum and Fischer, 2006; Wong et al, 2004).
Terminology
Definitions
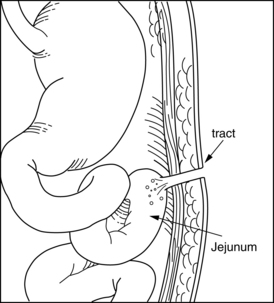
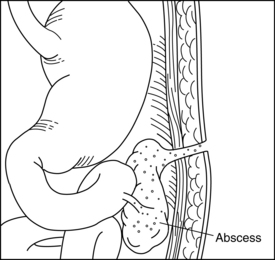
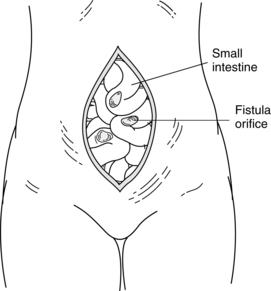
Fistula is a Latin word meaning “pipe” or “flute.” A fistula is an abnormal passage between two or more epithelialized surfaces so that a communication tract develops from one body cavity or hollow organ to another hollow organ or to the skin (Figures 38-1 to 38-3). Thus, a gastrointestinal fistula communicates between the lumen of the gastrointestinal tract and another organ. An enterocutaneous fistula communicates specifically between the lumen of the gastrointestinal tract and the skin. A draining wound, surgically placed drain site, or wound dehiscence should not be misinterpreted as a fistula.
Classification
Several methods are used to classify fistulas. These classification schemes are useful in predicting the morbidity rate, mortality rate, and potential for spontaneous closure (Wong et al, 2004).
From an anatomic perspective, the fistula may be simple or complex (Box 38-1). The simple fistula has a short, direct tract, no abscess, and no other organ involved, whereas the complex fistula is associated with an abscess, has multiple organ involvement, and may open into the base of a wound.
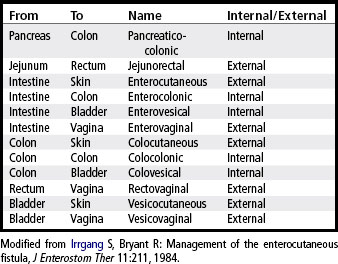
A fistula may be classified according to location. The internal fistula exists between internal structures, whereas the external fistula communicates between an internal organ and the skin, vagina, or rectum. This classification system is more specific in that the site of origin and site of termination are identified. Examples of such terminology are listed in Table 38-1.
A fistula may be classified according to volume of output (Table 38-2). A high-output fistula is most commonly defined as producing more than 500 ml per 24 hours, a moderate-output fistula is associated with output of 200 to 500 ml per 24 hours, and a low-output fistula produces less than 200 ml per 24 hours (Maykel and Fischer, 2003; Nussbaum and Fischer, 2006). The frequencies of sepsis, malnutrition, and fluid and electrolyte imbalance are directly related to fistula output. Fistula output is a direct reflection of the fistula’s site of origin. Distal large bowel fistulas typically have low output (<200 ml per 24 hours), whereas most proximal small bowel fistulas drain at least 1,000 to 1,500 ml per 24 hours initially and are considered high output. The high-output fistula is associated with severe malnutrition, significant fluid and electrolyte disturbance, higher morbidity and mortality rates, and lower spontaneous closure rate (Kassis and Makary, 2008; Wong et al, 2004).
TABLE 38-2 Fistula Classification
| Designation | Characteristics | |
|---|---|---|
| Location | Internal | Tract contained within body |
| External | Tract exits through skin | |
| Involved structures | Colon | Colon |
| Entero- | Small bowel | |
| Vesico- | Bladder | |
| Vaginal | Vagina | |
| Cutaneous | Skin | |
| Recto- | Rectum | |
| Volume | High-output | >500 ml per 24 hours |
| Moderate-output | 200–500 ml per 24 hours | |
| Low-output | <200 ml per 24 hours |
Modified from Boarini J et al: Fistula management, Semin Oncol Nurs 2:287, 1986.
Manifestations
Fever and abdominal pain are the initial indicators of a possible fistula (Nussbaum and Fischer, 2006). The passage of gastrointestinal secretions or urine through an unintentional opening onto the skin heralds the development of a cutaneous fistula. Manifestations of a fistula exiting through the vagina (i.e., rectovaginal or vesicovaginal) include passage of gas, feces, purulent material, or urine through the vagina, discharge that is extremely malodorous. Irradiation-induced rectovaginal fistulas often are preceded by diarrhea, passage of mucus and blood rectally, a sensation of rectal pressure, and a constant urge to defecate (Saclarides, 2002). Fistulas between the intestinal tract and the urinary bladder (e.g., colovesical fistula) present with passage of gas or stool-stained urine through the urethra.
Medical management
The desired endpoint of the medical management of a fistula is spontaneous closure. Approximately 19% to 40% of all fistulas will close spontaneously with conservative medical management, but only when sepsis is controlled and nutrition support is adequate and appropriate (Kassis and Makary, 2008). Of the fistulas that do heal spontaneously, 80% to 90% do so within 5 weeks, provided the patient is adequately nourished (Kassis and Makary, 2008; Maykel and Fischer, 2003). Factors known to correlate with spontaneous fistula closure include absence of sepsis, adequate nutritional support, low output, and classification as a postoperative fistula (Campos et al, 1999; Nussbaum and Fischer, 2006). Approximately 90% of simple type 1 fistulas close spontaneously, whereas less than 10% of complex type 2 fistulas close spontaneously (Wong et al, 2004).
Nonsurgical treatment
Objective 2: Control of Infection. Sepsis is the major cause of death in patients with enteric fistulas. The bacteria proliferate rapidly in the poorly vascularized tissue typically surrounding a fistula tract (Kassis and Makary, 2008). Pooling of bowel contents as a result of the dehiscence of a suture line precipitates localized and then diffuse abdominal pain, ileus, fever, and, ultimately, septic shock. The presence of systemic or local sepsis must be evaluated, typically with computed tomographic scanning or ultrasound. Effective drainage can be accomplished by use of percutaneous radiographic techniques or surgery. The specific approach depends on abscess location, patient status, and available resources. Surgical laparotomy for control of sepsis should be limited to proximal diversion and drainage of the abscess. Definitive repair of the fistula is undertaken at a later time only after inflammation has receded and nutrition is restored. Abscess contents should be cultured (aerobic and anaerobic) and Gram stained to identify the causative organisms and sensitivities. Organisms are most commonly of bowel origin: coliform, bacteroides, and enterococci. Staphylococci also may be present. Antibiotics are only appropriate in the presence of an infection and in conjunction with adequate drainage of the abscess (Wong et al, 2004).
Intestinal output must be minimized. Conventional methods for achieving this goal are giving the patient nothing by mouth (NPO) and administering histamine (H2) receptor antagonists. NPO status decreases luminal contents, gastrointestinal stimulation, and pancreaticobiliary secretion. Administering H2 antagonists (e.g., cimetidine) prevents stress ulcerations and decreases gastric, biliary, and pancreatic secretions. Despite reducing gastrointestinal secretions, H2 receptors have not been shown to speed closing of the enterocutaneous fistula (Maykel and Fischer, 2003).
Although not a standard in routine fistula care, administration of somatostatin-14 has been shown to further decrease intestinal output in some situations. This naturally occurring hormone is present throughout the body; more than two thirds is derived from the gastrointestinal tract, especially the distal portion of the stomach, duodenum, jejunum, and pancreas. In the presence of fat, protein, and carbohydrates with a meal, somatostatin secretion significantly increases. Somatostatin release is inhibited by the release of acetylcholine from cholinergic neurons (Nussbaum and Fischer, 2006). It has extensive, well-known biologic effects that include inhibition of gastric, biliary, pancreatic, and salivary secretions and reduced gastrointestinal motility, gastric emptying, and gallbladder emptying. When used in combination with total parenteral nutrition (TPN), a synergistic effect on reduced gastrointestinal secretions can be expected. Fistula output reductions of at least 50% within 24 hours and a significant reduction in the time to spontaneous closure of the fistula have been reported (Fagniez and Yahchouchy, 1999; Hesse et al, 2001). The short half-life of 1 to 2 minutes requires that somatostatin-14 be administered through continuous intravenous infusion (250 mcg/hr). The analogue octreotide has a half-life of almost 2 hours, so it can be administered three times daily subcutaneously (300 mcg/day). Results from available prospective controlled studies and randomized controlled studies of octreotide have been mixed. Overall it appears to have similar but less dramatic reduction of output and closure rates (Makhdoom et al, 2000). The most encouraging results of octreotide are seen with postoperative, high-output small bowel fistulas in which fluid-, electrolyte-, and protein-store depletion is prevented. These medications are less effective with fistulas associated with intrinsic bowel diseases, such as ulcerative colitis or Crohn’s Disease (Hild et al, 1986). Somatostatin or octreotide administration should be stopped if fistula output does not decrease in the first 48 hours of treatment or if no response is seen after 2 to 3 weeks of treatment, respectively.
Maykel and Fischer (2003) recommend caution when using somatostatin. They report that because use of somatostatin precipitates villous atrophy, interruption of intestinal adaptation, and acute cholecystitis, somatostatin should not be used routinely for treatment of the enterocutaneous fistula.
Adequate nutritional support is achieved when the patient is maintained in a state of positive nitrogen balance and receives adequate vitamin and trace mineral replacement. The amount of calories and protein required will depend on the patient’s preexisting status, sepsis, and fistula output. Caloric needs range from 30 to 40 kcal per kilogram per 24 hours; the goal should be a calorie-to-nitrogen ratio of 150:1. Protein requirements are estimated at 1.5 to 1.75 g per kilogram per 24 hours or 0.25 to 0.35 g of nitrogen per kilogram body weight per 24 hours (Wong et al, 2004). It is important to initiate nutritional support without delay because lean body mass is lost at a rate of 300 to 900 g per 24 hours depending upon the degree of stress (Maykel and Fischer, 2003). Trace elements (e.g., copper, zinc, magnesium), multivitamins, and vitamins (B, C, K) must be supplemented (Maykel and Fischer, 2003; Wong et al, 2004). González-Pinto and Moreno Gónzález (2001) recommend twice the recommended daily allowance (RDA) for vitamins and trace minerals and up to 10 times the RDA for vitamin C.
Interest in the use of enteral nutrition has resurfaced because of the recognition that even small amounts of enteral nutrition will maintain the normal structural, immunologic, and hormonal integrity of the gastrointestinal tract and prevent translocation of bacteria. However, enteral nutrition requires approximately 4 feet of small intestine (Knechtges and Zimmermann, 2009; Maykel and Fischer, 2003). Enteral nutrition is appropriate when fistulas are located in the most proximal or distal portion of the gastrointestinal tract; however, the gastrointestinal tract must be functional and the patient cooperative. Many types of enteral solutions are available, and a dietician should be consulted to recommend the most appropriate solution and administration procedure so that gastrointestinal intolerance (e.g., diarrhea, abdominal distention) can be avoided.
Objective 5: Definition of Fistula Tract. After the patient is stabilized (fluid and electrolytes balanced and infection controlled), the fistula must be examined to ascertain (1) the origin of the fistula tract, (2) the condition of adjacent bowel, (3) the presence of additional abscess pockets, and (4) the presence of distal obstruction or bowel discontinuity. Water-soluble contrast agents (e.g., Renografin, Hypaque, Gastrografin) are preferred to visualize the fistula tract and are administered through the fistula orifice using a soft-tip catheter. A computed tomographic scan is indicated only when the patient is not responding to conservative treatment. If other organs are involved, additional tests (e.g., cystoscopy or intravenous pyelogram) should be pursued (Wong et al, 2004).
Fistula closure is sometimes successful with the insertion of clotting substances into the fistula tract. The concept of using fibrin for anastomosis of tissue was first used for hemostasis in 1909 (Migaly and Rolandelli, 2006). Fibrin sealants (also referred to as fibrin glue) are composed of a concentrated allotment of fibrinogen/factor XIII/fibronectin and thrombin that congeals to form an insoluble fibrin clot when mixed with calcium chloride, a process that essentially replicates the last step of the coagulation cascade (Kassis and Makary, 2008; Tran and Thorson, 2008). The mechanism of action consists of fibrin glue providing a “matrix” for the influx of various cells and collagen formation (Buchanan et al, 2003).
Fibrin sealant products can be created from the patient’s own plasma (autologous) or from human plasma that has been collected and pooled from many donors (heterologous) after screening and viral testing. The fibrin glue is applied endoscopically at the origin of the fistula to seal the fistula (Migaly and Rolandelli, 2006). Although fibrin sealant products appear to have a role in the management of low-output fistulas (e.g., perirectal), the role of fibrin glue relative to the complexity of the fistula and timing of the procedure has yet to be determined.
Surgical treatment
Surgical intervention to close the fistula will be required when impediments to spontaneous closure have been identified. Factors known to prevent spontaneous closure are listed in Box 38-2 (Nussbaum and Fischer, 2006). If any of these factors are present and closure of the fistula is the ultimate goal for the patient, a surgical intervention will eventually be necessary. Surgical procedures may also be indicated for palliation.
BOX 38-2 Factors that Prevent Spontaneous Fistula Closure
• Compromised distal suture line/anastomosis (i.e., tension on suture line, improper suturing technique, inadequate blood supply to anastomosis)
• Foreign body in fistula tract or suture line
• Epithelium-lined tract contiguous with skin
• Presence of tumor or disease in site
• Previous irradiation to site
The exact timing for surgical intervention is variable, depending on the patient’s status. In general, operative interventions to close the fistula tract should be delayed until the patient is in optimum condition (i.e., positive nitrogen balance and control of infection are established). If the patient is nutritionally and metabolically stable, definitive surgery for a simple or complex type 1 fistula is appropriate when a persistently draining fistula is in a sepsis-free environment for 6 to 8 weeks. It is important that definitive surgery be delayed until the abdominal wall is soft and supple; tissues should return to a normal soft, pliable state, particularly in the presence of irradiated tissue (González-Pinto and Moreno Gónzález, 2001; Wong et al, 2004).
Complex type 2 fistulas invariably require definitive surgery; however, the timing of surgery is not well defined. Nutritional status, metabolic status, and immunocompetence should be normalized, and the obliterative peritonitis and inflammation associated with chronic peritoneal contamination should be resolved (Wong et al, 2004). Judicious timing of surgery for complex type 2 fistulas is warranted. The most often reported timing ranges from 3 to 6 months.
The surgical interventions available for management of enterocutaneous fistulas will either divert the gastrointestinal tract (without resection of the fistula) or provide definitive resection of the fistula tract (Nussbaum and Fischer, 2006). Factors such as location, size, and cause of the fistula, the patient’s overall status, and the presence of irradiated tissue will influence the approach selected. However, Maykel and Fischer (2003) warn that the best results occur with resection of the fistula and end-to-end anastomosis and that other surgical procedures represent a compromise.
Enteric fistulas communicating with the urinary tract will always require diversion of the fecal stream proximal to the fistula site to prevent urinary tract infections and pyelonephritis.
Nursing management
Goals
Effective nursing management of the enterocutaneous fistula strives to achieve the eight goals listed in Box 38-3. Optimally, all the goals are achieved simultaneously; however, that is not always possible, and prioritizing is frequently necessary. For example, a pouching system may effectively contain output and odor as well as provide significant skin protection. However, complete mobility may not be possible, or the pouching system may be expensive. Interventions to achieve the goals begin as soon as the patient is noted to have a fistula; they are not contingent upon medical diagnosis.
Four general principles should guide the care of the patient with a fistula (Rolstad and Wong, 2004):
Assessment
The method selected to manage a fistula is guided by the assessment of the four key fistula characteristics outlined in Box 38-4. Because fistulas change in shape and contour over time, repeat assessment and monitoring are necessary. Modifications to the initial containment system are invariably necessary (Scardillo and Folkedahl, 1998; Wiltshire, 1996; Zwanziger, 1999).
BOX 38-4 Fistula Assessment and Documentation Guide
1. Source (e.g., small bowel, bladder, esophagus)
2. Characteristics of effluent
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree