26 Key Points 1. Careful examination of the lowest sacral segments is necessary to identify neurological injury and determine prognosis in thoracolumbar burst fractures with neurological injury. 2. Neurological recovery varies with the anatomical structure at the site of the fracture (spinal cord, conus medullaris, or cauda equina). It may also be influenced by timely stabilization and decompression. 3. Early posterior stabilization and realignment may be followed by anterior decompression at a later date (within the first 1 to 2 weeks following injury) if ongoing bony or soft tissue compression of neurological elements persists and is demonstrated on imaging. 4. Profound neurological injury may lead to “walking paraplegia,” wherein the patient regains the ability to ambulate but is plagued by significant weakness and poor balance, as well as profound bladder, bowel, and sexual dysfunction. The thoracolumbar region comprises the 11th and 12th thoracic, and first and second lumbar, vertebrae (T11 to L2). At these motion segments, the rigid thoracic kyphosis transitions into the mobile lumbar lordosis, making this region susceptible to traumatic injuries.1–4 The patterns of injury to the spinal column include compressive injuries, such as burst fractures of the vertebral body, as well as distraction (Chance-type fractures) and translational/rotational injuries, such as fracture-dislocations.5–7 There are also a variety of neurological structures that can be injured, including the lower portion of the thoracic spinal cord, the conus medullaris, and the cauda equina. This chapter describes the nature, evaluation, and management of compressive burst fractures that result in neurological injury to the distal spinal cord, conus medullaris, and cauda equina. The complexity of the neuroanatomy in this region influences the prognosis and should influence therapeutic decision making as well. The spinal cord terminates as the conus medullaris in a variable location relative to the vertebral segments.8,9 Although there is no defined anatomical landmark that identifies the distal extent of the conus medullaris, its tip has a variable location between the T11–12 disk space and the L4 vertebra, with its most common location being the L1–2 disk space. The conus medullaris morphologically represents a transition from the central to the peripheral nervous system.10 At the T12–L1 disk space, the spinal cord tapers and the L1–5 nerve roots form a peripheral rim around the distal spinal cord. At the L1–2 disk space, the lumbar nerve roots are somatotopically oriented from lateral (L2) to medial (L5).11–13 The lumbar sympathetic, sacral parasympathetic, and sacral somatic nerves originate within the conus medullaris and are carried within the nerve roots of the cauda equina. Although many of these lumbar roots descend over several vertebral segments within the thecal sac, the formal designation of the cauda equina begins below the termination of the conus medullaris. One of the unique features of the thoracolumbar region is the disparity between the location of the spinal cord segment and the vertebrae; for example; the L5 vertebra is very distant from the segment of the spinal cord from which the L5 dorsal and ventral roots emanate. The very practical impact of the variation in segmental location and variable neuroanatomy is that the description of a burst fracture resulting in a neurological injury at the L1 vertebral level tells us very little about the precise neurological structure that has been injured. Thoracolumbar burst fractures account for up to 17% of major spinal fractures, and males are at a fourfold greater risk than females. The incidence of neurological deficit resulting from thoracolumbar burst fractures is estimated to be 50 to 60%.3,4,14 Neurological recovery from injury to the conus medullaris or cauda equina is unpredictable. Variables that influence prognosis are thought to include age, comorbidities that influence vascularity (diabetes, etc.), magnitude of energy absorbed, secondary injuries, and possibly the timing of neural decompression.1,15–24 Traumatic lesions of the cauda equina causing sudden, acute neurological deterioration generally have a poorer prognosis when compared with the gradual onset of lower motor neuron dysfunction in chronic or acute nontraumatic cauda equina syndrome. Neurological recovery for injuries to the termination of the spinal cord, and particularly to the cauda equina, carries a better prognosis than does recovery for injuries to the midthoracic spinal cord.25 In conus medullaris injuries, if there is some residual motor sparing, it is most likely to occur in the more proximal lower extremity muscle groups (hip flexors, adductors, and knee extensors) because these more cephalad nerve roots will be most likely to have escaped injury. Greater motor score improvement in patients with spinal cord injuries compared with conus medullaris and cauda equina injuries has been reported by Kaneda et al., who also noted the highest final motor scores in the cauda equina patients. The dramatic neurological recovery reported in Kaneda et al.’s series has not been duplicated.26,27 Very little is known about the specific motor recovery patterns in lower thoracic spinal cord, conus medullaris, and cauda equina injuries and how patient factors, injury patterns, and perioperative variables affect neurological recovery. In a recent study from our center,25 the neural axis level of injury, identified with magnetic resonance imaging (MRI), and not the vertebral column level of injury, was predictive of neurological motor recovery. The initial motor score also influenced recovery. Identification of the precise level of neural axis injury utilizing MRI was one of the key determinants with respect to the prognosis for patients that sustain these common injuries. A thorough Advanced Trauma Life Support (ATLS) approach is required when evaluating these patients, and a precise neurological exam is critical for characterizing the type of clinical syndrome that the patient has sustained.28,29 The neurological structures at the level of the thoracolumbar spine are critical for lower extremity motor and sensory function as well as bowel, bladder, and sexual function. Examination may reveal variable lower extremity weakness, absent lower limb reflexes, and anesthesia ranging from the waistline down to the lowest sacral segments in the perianal region. Preservation or early return of the bulbocavernosus reflex (BCR) and anal reflex is more commonly observed with spinal cord injuries, whereas they are typically permanently abolished with either conus or cauda injuries. Because of this, injuries of the cauda equina cannot be strictly assigned an American Spinal Injury Association (ASIA) grade.30 Cauda equina injuries are pure lower motor neuron injuries, and as such they are associated with absent deep tendon reflexes and BCR, a flaccid urinary bladder, and flaccid lower extremity paralysis. Although plain films provide useful information, particularly to assess the effects of gravity on spinal alignment in patients without a neurological deficit and questionable stability, their utility in the evaluation of an acute neurological deficit at the thoracolumbar spine is superseded by advanced imaging modalities. A computed tomographic (CT) scan with sagittal and coronal reformats is crucial for characterizing the bony injury as well as the resultant bony canal compromise (Fig. 26.1). There is no difference in neural recovery based on the final spinal canal area or spinal level of injury (T11–L2).31 No association between initial canal encroachment and neurological recovery has been shown.21,32,33 Fig. 26.1 Paramedian reformatted CT scans from a 56-year-old male with an L1 burst fracture. In the setting of a neurological deficit attributable to a traumatic thoracolumbar spine injury, MRI is almost always advisable. MRI may not be appropriate, due to medical instability, patient size, or other contraindications related to metallic implants. The value of MRI includes the assessment of spinal cord signal change, the precise location of the conus medullaris, and evaluation of the posterior ligamentous complex (PLC).7,34 The integrity of the PLC is an important consideration in the management of thoracolumbar burst fractures, and it is best evaluated with short tau inversion recovery (STIR) or fat-suppressed T2 sequences on MRI (Fig. 26.2). MRI may also be of value in anticipating treatment complexities, such as identifying the presence and location of disk or bone fragments in the spinal canal; anticipating the location of maximal spinal cord compression, traumatic durotomy, and cerebrospinal fluid (CSF) leaks; and planning surgical approaches. The extent of abnormal signal change in the spinal cord may have prognostic significance. The most common spinal column injuries resulting in codus medullaris injury (CMI) or cauda equina injury (CEI) are burst fractures (Fig. 26.3) and fracture-dislocations. Flexion-distraction injuries may also lead to neurological deficits at these levels, although they are less common and have less risk of an associated neurological lesion.1,3,4,10 Combining the assessment of spinal stability, neurological status, and unique patient factors, the surgeon is now able to develop an appropriate management plan. Fig. 26.2 Magnetic resonance imaging revealing increased signal change and swelling in the conus medullaris and terminal spinal cord and posterior ligament injury. Fig. 26.3 Magnetic resonance imaging of an L2 burst fracture demonstrating bony compression of the cauda equina. In the setting of CMI and CEI secondary to spinal column trauma, the treating surgeon must decide whether surgical treatment is indicated and, if so, what the surgical plan should entail. Ideally, the analysis should be evidence based and consider the balance of harms, benefits, and costs of a proposed intervention. Treatment options include bed rest, orthoses, and various surgical approaches, construct lengths, and instrumentation alternatives.4,28,35–42 In the case of traumatic CMI or CEI where the patient will likely benefit from early expert management related to bowel and bladder function, treatment in a specialized center may be of benefit. A review of published literature involving traumatic neurological deficits at the thoracolumbar and lumbar spine reveals generally low-quality retrospective studies of heterogeneous patients and treatment approaches. Results specifically cited as relating to CMI or CEI may not be precisely applicable to these injuries because these syndromes are typically inferred based on the spinal level of injury or the neurological presentation. A systematic review on the effectiveness of surgical decompression for thoracolumbar burst fractures with a neurological deficit showed a weak trend toward improved recovery in the nonsurgical group but involved heterogeneous surgical techniques.16 Patients with incomplete neurological deficits fared better with surgical stabilization and decompression. Based on the population-based study by Daniels et al. it would appear that patients with traumatic CMI, CEI, and spinal cord injuries are frequently treated non-surgically.37 Only 61.4% of patients with a thoracolumbar fracture and a neurological injury are treated surgically. The percentage is only slightly greater in the highest-volume centers. Daniels et al.’s conclusions are drawn from a review of treatment codes and may be inherently flawed due to the potential inaccuracies in this type of administrative data. This literature supports the assertion that nonsurgical treatment is a viable alternative. Several studies have shown no correlation between neurological recovery and nonoperative or surgical treatment, canal compromise, or fracture pattern.22,23,43,44 Other authors have suggested that patients with neurological deficits secondary to thoracolumbar and lumbar spinal injuries may benefit from surgical treatment in terms of shorter hospital stays, which often leads to more timely active rehabilitation.17,38,45,46 It is the authors’ opinion that the specific expertise and resources necessary to provide safe, effective nonoperative care to patients with a spinal cord injury are diminishing, particularly in North America and Europe. Although nonsurgical care of traumatic CMI and CEI will likely result in some degree of neurological improvement, the vast majority of these injuries should be treated with surgical stabilization and, when necessary, with a concomitant decompression. Not only is this likely to reduce the patient’s hospital stay and facilitate nursing and rehabilitation but also it is clearly safe from a neurological perspective and may optimize neurological recovery. Studies of anterior decompression for thoracolumbar fractures with incomplete neurological deficits have demonstrated that neurological recovery was not associated with the timing of anterior decompression. Generally, patients improve by at least one motor grade after even delayed decompression, particularly those with CEI. There also appears to be substantial improvement in bladder function following decompression for injuries at T12–L1.26,47,48 The quality of decompression may be improved in patients treated with anterior decompression, and this may influence bladder/bowel recovery. Decompression and stabilization, as opposed to posterior fusion alone, appear to lead to a more substantial motor improvement in patients with lumbar fractures and incomplete cauda equina neurological deficits.49 Other authors report satisfactory neurological outcomes with motor improvement in up to half of the patients and bladder functional improvement in two thirds, suggesting that posterior surgery alone is safe and acceptable in patients with CMI and CEI. Delayed anterior vertebrectomy can often be performed within a subacute timeframe depending upon stability or neurological indications.50,51 Most studies looking at the timing of surgery are underpowered, and the only conclusions that can be drawn are that it is likely safe to perform early surgery and early surgery does not appear to be associated with a profound risk of neurological deterioration.20,52 There is some suggestion that early fixation results in improved clinical outcome in terms of complications and hospital stay, but it has an unclear effect on neurological outcome.52,53 Lower spinal cord injuries result in an upper motor neuron spastic bladder where the sacral micturition center and sacral reflex arcs continue to respond, although they are deprived of brain stem and cortical micturition control. This syndrome may lead to detrusor hyperreflexia, increased pressure within the bladder, and the potential risk of retrograde urine flow.52,54–57 Injuries to the cauda equina, on the other hand, result in a lower motor neuron flaccid bladder, as do injuries located within the conus medullaris. Lower motor neuron disruption typically results in a flaccid bladder, urinary retention, and overflow incontinence. Treatment consists of clean intermittent catheterization to ensure complete bladder emptying. Management goals include avoidance of bladder overdistension and retrograde urine flow, which may lead to pyelonephritis and secondary renal failure. With respect to sexual function, men with a lower motor neuron lesion will have more difficulty achieving a reflexive erection. The pharmacological treatment of erectile dysfunction is dependent on the level and extent of the spinal cord injury. Fertility issues, for female and male patients, should be specifically addressed by urologists and gynecologists with a specific interest in spinal cord injury because most patients will retain the ability to have children. Thoracolumbar burst fractures may be associated with injury to the lower spinal cord, the conus medullaris, or the cauda equina. When injured, each of these neuroanatomical structures carries with it a unique spectrum of functional impairment and prognosis for neurological recovery. Greater motor score improvement occurs following cauda equina injury than following a low thoracic spinal cord injury. Other predictors of favorable neurological recovery include the presence of sacral sensation and higher initial motor score at the time of initial assessment. From a prognostic point of view, it is important to visualize the exact location of the conus medullaris in relation to the level of injury and report on the MRI-determined neural axis level of injury as opposed to the vertebral level of injury. Surgical decompression for incomplete thoracolumbar injuries has become common practice and is supported by poor quality evidence in the literature. Non-surgical treatment results in neurological improvement as well; however, the comparative efficacy or effectiveness of surgery and nonsurgical treatment cannot be assessed by the available evidence specific to CMI or CEI. Regardless of the potential influence of surgical treatment on neurological improvement, surgical treatment may be preferred due to shorter hospital stays, earlier rehabilitation, availability of equipment, and expert nursing care, as well as patient preference. When surgical treatment is selected, posterior stabilization with or without posterolateral decompression appears to offer good neurological outcomes and has the benefit of being familiar to the surgeon and avoiding the additional morbidity of an anterior approach. Anterior decompression, however, may offer potential benefits in terms of bladder recovery, and it can always be performed in a sub-acute time frame after initial posterior stabilization and after the patient is less acute. Indications for a secondary anterior decompression and stabilization would include (1) stability in those patients who have extremely comminuted fractures and (2) persistent neural element compression after posterior surgery, particularly in the presence of a profound or incomplete neurological deficit. From the available evidence, timing of surgery for traumatic CMI or CEI does not improve neurological recovery, although there does not appear to be any evidence of a deleterious effect from early surgical stabilization or decompression. Pearls
Management of Acute Spinal Cord Injury in Thoracolumbar Burst Fractures Including Cauda Equina Syndrome
 Neuroanatomy
Neuroanatomy
 Demographics
Demographics
 Natural History of Conus and Cauda Injuries
Natural History of Conus and Cauda Injuries
 Clinical and Neurological Evaluation
Clinical and Neurological Evaluation
 Spinal Imaging
Spinal Imaging
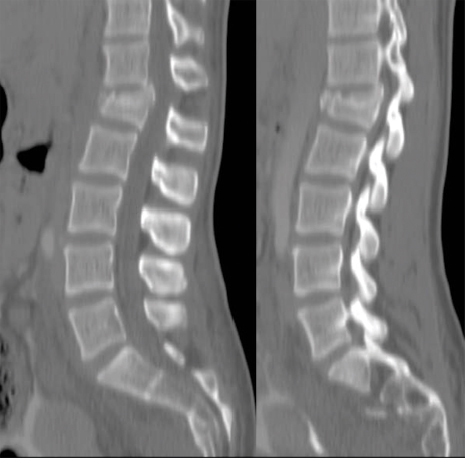
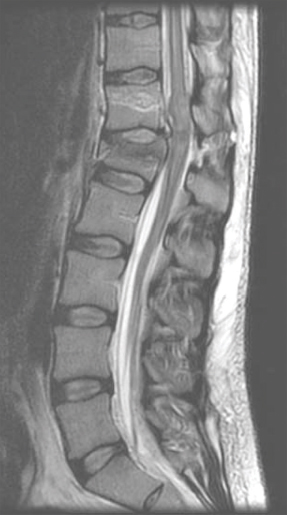
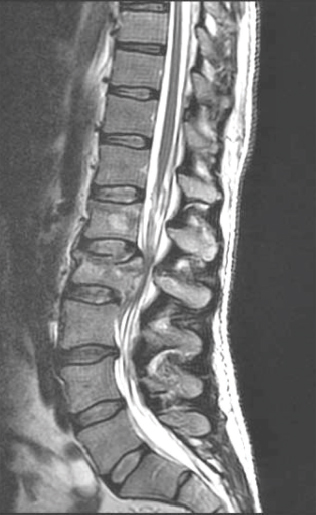
 Factors Influencing Clinical and Neurological Outcome
Factors Influencing Clinical and Neurological Outcome
Neurological Outcome and Operative versus Nonoperative Treatment
Neurological Outcome and Surgical Approach/Timing
Neurological Outcome and Bladder/Sexual Function
 Conclusion
Conclusion
 Thoracolumbar injuries have a variable prognosis that is dependent upon the neuroanatomical structure located at the level of the fracture.
Thoracolumbar injuries have a variable prognosis that is dependent upon the neuroanatomical structure located at the level of the fracture.
 Sacral sensory preservation and injury at the level of the cauda equina are favorable prognostic indicators.
Sacral sensory preservation and injury at the level of the cauda equina are favorable prognostic indicators.
 In the severely traumatized patient, posterior stabilization can be performed to facilitate nursing care, and then the patient can be reimaged with MRI or CT scanning to assess the need for a subacute anterior vertebral body re-section and reconstruction.
In the severely traumatized patient, posterior stabilization can be performed to facilitate nursing care, and then the patient can be reimaged with MRI or CT scanning to assess the need for a subacute anterior vertebral body re-section and reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


