Chapter 13 Macroreentrant Atrial Tachycardia (“Atypical Atrial Flutter”)
Pathophysiology
Atypical Isthmus-Dependent Right Atrial Macroreentry
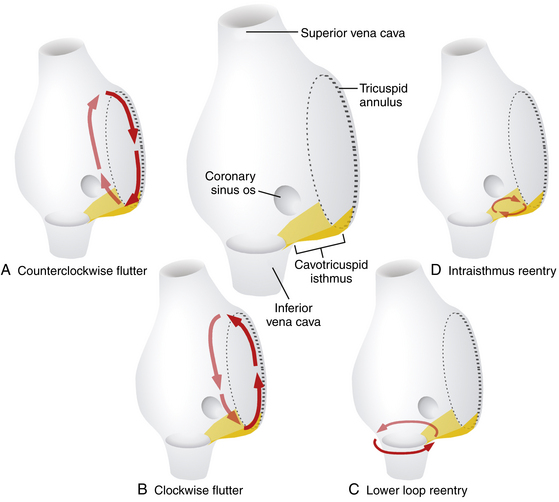
Lower loop reentry and intraisthmus reentry are macroreentrant circuits that are confined to the right atrium (RA) and incorporate the CTI as a critical part of the circuit. However, in contrast to typical AFL, the circuit is not peritricuspid (Fig. 13-1). Nevertheless, because the CTI is still a necessary part of the circuit, these arrhythmias are amenable to CTI ablation, as is true for patients with typical AFL.1
Lower Loop Reentry
Lower loop reentry is a form of CTI-dependent AFL with a reentrant circuit around the inferior vena cava (IVC); therefore, it is confined to the lower part of the RA (see Fig. 13-1). It often coexists with counterclockwise or clockwise typical AFL and involves posterior breakthrough across the crista terminalis. Lower loop reentry can rotate around the IVC in a counterclockwise (i.e., the impulse within the CTI travels from the septum to the lateral wall) or clockwise fashion. A breakdown in the inferoposterior boundaries of the CTI produced by the eustachian ridge and lower crista terminalis causes the circuit to revolve around the IVC (instead of around the tricuspid annulus), across the eustachian ridge, and through the crista terminalis, with slow conduction because of transverse activation through that structure. Alternatively, the circuit can exit at the apex of Koch triangle and come behind the eustachian ridge to break through across the crista terminalis behind the IVC and then return to the CTI. This arrhythmia is usually transient and terminates by itself or converts spontaneously into AFL or atrial fibrillation (AF).1
Intraisthmus Reentry
Intraisthmus reentry is a reentrant circuit usually occurring within the region bounded by the medial CTI and coronary sinus ostium (CS os; see Fig. 13-1). Circuits in the lateral portion of the CTI can also occur but are less common. This arrhythmia can be sustained and usually occurs in patients who have undergone prior, and often extensive, ablation at the CTI. Intracardiac recordings usually resemble typical AFL. However, in this form of CTI-dependent AFL, entrainment pacing from the lateral CTI demonstrates a post-pacing interval (PPI) longer than the tachycardia CL, a finding indicating that the lateral CTI is not part of the reentrant circuit. On the other hand, pacing from the region of medial CTI or CS os demonstrates concealed entrainment with PPI equal to the tachycardia CL. Fractionated or double potentials usually can be recorded in this area and can be entrained. Although the anatomical basis of this arrhythmia remains unknown, a linear lesion across the medial CTI, usually at the site of a very prolonged electrogram, can cure the tachycardia.
Non–Isthmus-Dependent Right Atrial Macroreentry
Lesional Right Atrial Macroreentrant Tachycardia
Atrial macroreentry in the right free wall is the most common form of non–CTI-dependent RA MRAT. These circuits can propagate around a central obstacle of a low-voltage area or scar in the lateral or posterolateral RA wall, arising spontaneously or as a consequence of prior atrial surgery.The central obstacle of the macroreentrant circuit can be an atriotomy scar (in patients who have undergone surgery for congenital or valvular heart disease), a septal prosthetic patch, a suture line, or a line of fixed block secondary to radiofrequency (RF) ablation. Other obstacles can also include anatomical structures located in the vicinity of the scar (superior vena cava [SVC], IVC). Occasionally, these ATs are associated with areas of electrical silence, a finding suggesting atrial scarring in patients who have not undergone prior atrial surgery. These patients have a characteristic posterolateral and lateral distribution of RA scarring and frequently have more than one tachycardia mechanism. Low-voltage electrograms characterizing areas of scar and double potentials characterizing a line of block can be observed during both normal sinus rhythm (NSR) and AT. In one study, electroanatomical mapping revealed that RA electrically silent areas (electrogram amplitude 0.03 mV or lower) were involved in the tachycardia mechanism in two thirds of the patients with non–CTI-dependent RA MRAT.2,3
Patients with congenital heart disease have a high prevalence of AT, particularly after they have undergone reparative or palliative surgical procedures. For MRATs in adults with repaired congenital heart disease, three RA circuits are generally identified: lateral wall circuits with reentry around or related to the lateral atriotomy scar, septal circuits with reentry around an atrial septal patch, and typical AFL circuits using the CTI. These arrhythmias are discussed separately in Chapter 14.1,4
Upper Loop Reentry
This type of MRAT involves the upper portion of the RA, with transverse conduction over the crista terminalis and wavefront collision occurring at a lower part of the RA or within the CTI. When upper loop reentry was first reported, it was thought to be a reentrant circuit using the channel between the SVC, fossa ovalis, and crista terminalis. Noncontact mapping studies have shown that this form of MRAT employs the crista terminalis as its functional central obstacle. The impulse rotating in the circuit can be in a counterclockwise or clockwise direction. The CTI is not an intrinsic part of the reentrant circuit. Upper loop reentry can be abolished by linear ablation of the gap in the crista terminalis.5,6
Dual-Loop Reentry
Although RA MRAT (lesional RA macroreentry or upper loop reentry) can manifest as an isolated arrhythmia (single-loop reentry), it can occur in conjunction with typical clockwise or counterclockwise AFL, or both, as well as lower loop reentry. When two atrial macroreentrant circuits coexist and use neighboring anatomical structures, they create the so-called dual-loop reentry. Not uncommonly, ablation of one tachycardia results in transition to the other, and ablation of both circuits is necessary for clinical success. Detection of this change requires careful attention to the atrial activation sequence and ECG pattern after each RF application. The recording of multiple simultaneous electrograms, as continuous endocardial references, facilitates detection of these activation changes. Fusion between two simultaneous left atrial (LA) macroreentrant circuits is possible after prior AF ablation.7
Left Atrial Macroreentry
LA MRATs are less common than typical AFL and are frequently related to or coexist with AF. LA MRAT is a known complication of surgical and catheter-based therapies of AF, and it can occur in up to 50% of patients following extensive catheter ablation strategies (see Chap. 15).8 Additionally, cardiac surgery involving the LA or atrial septum can produce different LA macroreentrant circuits. However, LA circuits also can be found in patients without a history of atriotomy or prior ablation. Electroanatomical maps in the latter group often show low-voltage or areas of scar in the LA, which act as a central obstacle or barrier in the circuit. These areas are typically located at the posterior wall (45%), superior region (roof, 28%), or anteroseptal region (27%) of the LA. The pathogenesis of these areas with no electrical signals is not well established. Potential causes include volume and pressure overload (mitral valve disease, hypertension, heart failure), ischemia (atrial branch occlusion), postinflammation scarring (after myocarditis), atrial amyloidosis, atrial dysplasia, and tachycardia-related structural remodeling. These macroreentrant circuits show considerable anatomical variability and frequently involve multiple simultaneous loops.1,2
Perimitral Atrial Flutter
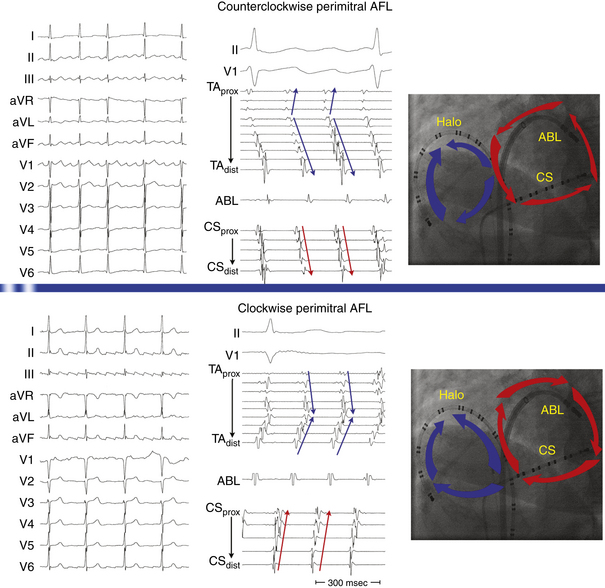
This circuit involves reentry around the mitral annulus in a counterclockwise or clockwise fashion (Fig. 13-2, see Video 12).  This arrhythmia is more common in patients with structural heart disease; however, it has been described in patients without obvious structural heart disease, but, in these patients, electroanatomical voltage mapping often shows scar or low-voltage areas on the posterior wall of the LA as a posterior boundary of this circuit. Perimitral MRAT is the most common MRAT in patients with prior LA ablation procedures for AF.
This arrhythmia is more common in patients with structural heart disease; however, it has been described in patients without obvious structural heart disease, but, in these patients, electroanatomical voltage mapping often shows scar or low-voltage areas on the posterior wall of the LA as a posterior boundary of this circuit. Perimitral MRAT is the most common MRAT in patients with prior LA ablation procedures for AF.
Left Septal Circuits
Left septal circuits represent a rare form of MRAT occurring in the absence of prior cardiac surgery. These circuits involve the LA septum primum, which acts as a central obstacle for the reentrant circuit. The right PV ostia serve as its posterior boundary, whereas the mitral annulus serves as its anterior boundary. Atrial dilation and concomitant antiarrhythmic drug therapy also seem to play a role by the prolongation of left intraatrial conduction, which then allows stable macroreentry circuits to persist.2 In patients with a history of surgery for atrial septal defects, scars or the patch on the septum can serve as the anatomical substrate of left septal circuits.
Clinical Considerations
Epidemiology
MRAT comprises a heterogeneous group of RA or LA macroreentrant circuits related to different anatomical and electrophysiological (EP) substrates. These arrhythmias are frequently associated with structural heart disease, congenital cardiac defects, previous cardiac surgical procedures, or surgical or catheter ablation procedures for AF. Often, these arrhythmias coexist with AF. However, MRAT occasionally occurs in a patient with no apparent structural heart disease.4
Although its exact prevalence among cardiac arrhythmias is difficult to establish clinically, MRAT is not a rare arrhythmia. It seems that the incidence of MRAT is increasing. This may be related to aging of the general population, which implies progressive alteration of the atrial electrical properties with development of an arrhythmogenic substrate, as well as to the increased numbers of surgical and catheter based procedures in the atria for treatment of AF.4,9
Principles of Management
With recent advances in the technology to visualize and modify the arrhythmia substrate, ablation of non–CTI-dependent MRAT has become increasingly successful, but it can be substantially more difficult than ablation of typical AFL. MRAT can have complex circuits that demand a thorough knowledge of atrial anatomy and a great deal of experience to correlate activation patterns with anatomical landmarks. When this type of AT is suspected, such as in patients with congenital heart disease who have had surgery, referral to an experienced center should be considered.4
Electrocardiographic Features
P wave morphology on the surface ECG is usually of limited value for precise anatomical localization of macroreentrant circuits. Analysis of the P wave can be impeded by partial or complete concealment of the P wave within the QRS complexes or T waves when the AT is associated with 1:1 or 2:1 AV conduction. Additionally, P wave morphology of a spectrum of RA and LA MRATs is highly variable. The presence of complex anatomy secondary to congenital abnormalities, prior atrial surgery, or a large low-voltage zone (secondary to underlying atrial substrate or extensive catheter or surgical atrial ablation) can modify atrial wavefront propagation in a nonuniform manner, resulting in deviated atrial activation vectors or low amplitude P waves. Furthermore, P waves produced by different underlying substrates may appear similar if the direction of activation of the atrial septum and LA is similar. The surface ECG morphology is most characteristic (and hence predictive) for establishing a diagnosis of counterclockwise typical AFL. Nevertheless, atypical ECG patterns have been described for typical AFL after AF ablation. Although clockwise typical AFL also has a characteristic appearance, this is more variable, and it can be mimicked by various other MRATs.5,10 On the other hand, AFL may show distinct isoelectric intervals between P waves, especially in the presence of extensive atrial scarring or a localized macroreentrant circuit, similar to focal AT.
Upper Loop Reentry
The surface ECG of upper loop reentry closely mimics that of clockwise typical AFL, with positive P waves in the inferior leads, because in most cases, both arrhythmias share a similar activation sequence of the LA, the septum, and caudocranial activation of the lateral RA. However, negative or isoelectric or flat P waves in lead I favor upper loop reentry over clockwise typical AFL. Conversely, positive P waves in lead I with an amplitude of more than 0.07 mV favor clockwise typical AFL. Additionally, the CL of upper loop reentry is usually shorter in comparison with typical AFL.5,6
Incisional Right Atrial Macroreentrant Tachycardia
The surface ECG morphology of free wall MRAT in a patient with a previous atriotomy is highly variable, depending on factors including the location of the scars and low-voltage areas, the direction of rotation, the presence of coexisting conduction block in the atrium, and the presence of a simultaneous typical AFL. The morphology of the atrial complex on the surface ECG can range from that similar to typical AFL to that characteristic of focal AT (see Fig. 14-5). Often, inverted P waves can be observed in lead V1. Depending on the predominant direction of septal activation, RA free wall MRAT can mimic either clockwise or counterclockwise typical AFL.5
Left Atrial Macroreentrant Tachycardia
The surface ECG morphology of LA MRAT caused by the different reentrant circuits is variable, and ECG findings are often similar for different underlying substrates, thus making the localization within the LA based on the ECG difficult. LA MRATs are usually associated with prominent positive P waves in lead V1 and upright (but frequently of low amplitude) deflections in leads II, III, and aVF. However, LA MRAT can result in ECG patterns of focal AT (discrete P waves and isoelectric baseline) because of a high prevalence of generalized atrial disease and slower conduction. Infrequently, LA MRAT can mimic typical AFL on the surface ECG.5
Perimitral Atrial Macroreentry
Most of these tachycardias show prominent forces in leads V1 and V2, with diminished amplitude in the inferior leads (see Fig. 13-2). It has been suggested that a posterior LA scar allows for domination by anterior LA forces. This constellation of findings may mimic counterclockwise or clockwise CTI-dependent AFL, but the decreased amplitude of frontal plane forces suggests an LA circuit. In patients with prior PV isolation procedures, the surface ECG morphology of counterclockwise perimitral MRAT can be different from that in patients without prior ablation, possibly related to varying degrees of prior LA ablation or scar. In these patients, counterclockwise perimitral MRAT demonstrates positive P waves in the inferior and precordial leads and a significant negative component in leads I and aVL. Furthermore, counterclockwise perimitral MRAT in these patients can have a morphology similar to that of left PV ATs. However, counterclockwise perimitral MRAT is suggested by a more negative component in lead I, an initial negative component in lead V2, and a lack of any isoelectric interval between P waves. Clockwise perimitral MRAT has limb lead morphology that is the converse of that of counterclockwise perimitral MRAT and an initial negative component in the lateral precordial leads. The positive P wave in leads I and aVL differentiates clockwise perimitral MRAT from counterclockwise CTI-dependent AFL and left PV AT.
Left Septal Circuits
Because the reentry circuit is on the septum, the surface ECG shows prominent, usually positive, P waves only in lead V1 or V2 and almost flat waves in most other leads (Fig. 13-3). This pattern can be caused by a septal circuit with anteroposterior forces projecting in lead V1 and the cancellation of caudocranial forces. This pattern was 100% sensitive for an LA septal circuit, but the specificity of this pattern for any type of LA MRAT was only 64%.
Electrophysiological Testing
Induction of Tachycardia
The programmed electrical stimulation protocol should include atrial burst pacing from the high RA and CS—down to a pacing CL at which 2:1 atrial capture occurs—and atrial extrastimulation (AES), single and double, at multiple CLs (600 to 300 milliseconds) from the high RA and CS (down to the atrial effective refractory period). Isoproterenol infusion (0.5 to 4 μg/min) is administered as needed to facilitate tachycardia induction. The goals of EP testing in patients with MRAT are listed in Table 13-1.
TABLE 13-1 Goals of Programmed Stimulation during Macroreentrant Atrial Tachycardia
AT = atrial tachycardia; CL = cycle length; LA = left atrium; RA = right atrium.
Tachycardia Features
MRAT is characterized by a constant CL, P wave polarity, morphology, and amplitude of the recorded bipolar electrograms and by the presence of a single constant macroreentrant circuit with a constant atrial activation sequence. The atrial activation sequence and atrial CL depend on the origin and type of the macroreentrant circuit. However, considerable variation in the atrial CL for a single macroreentry circuit is unusual, although it can be observed in the atrium contralateral to atrium of origin of the tachycardia. In contrast, focal ATs classically exhibit alterations in CL with speeding (warm-up) and slowing (cool-down) at the onset and termination of tachycardia. Variation in the AT CL of greater than 15% has been suggested as a reliable marker of a focal AT. However, a regular AT can be either focal or macroreentrant.11 Additionally, focal ATs often manifest as bursts of tachycardia with spontaneous onset and termination, although they can be incessant, and they may accelerate in response to sympathetic stimulus.5 Several criteria can help distinguish focal AT from MRAT (see Table 11-5).
Atrial activation during MRATs spans the whole tachycardia CL. In contrast, intracardiac mapping in the setting of focal ATs shows significant portions of the tachycardia CL without recorded atrial activity, and atrial activation time is markedly less than the tachycardia CL, even when recording from the entire cardiac chamber of tachycardia origin (see Fig. 11-2). However, in the presence of complex intramyocardial conduction disturbances, activation during focal tachycardias can extend over a large proportion of the tachycardia CL, and conduction spread can follow circular patterns suggestive of macroreentrant activation.5 On the other hand, long isoelectric intervals can occur between P waves during MRATs, especially when mapping is limited to only the atrium contralateral to the origin of the macroreentrant circuit or to only parts of the ipsilateral atrium; a focal activation can be observed, incorrectly suggesting a focal mechanism. This is particularly observed for LA MRATs in the presence of large areas of electrical silence. However, a thorough intracardiac activation mapping reveals atrial activation spanning the tachycardia CL.
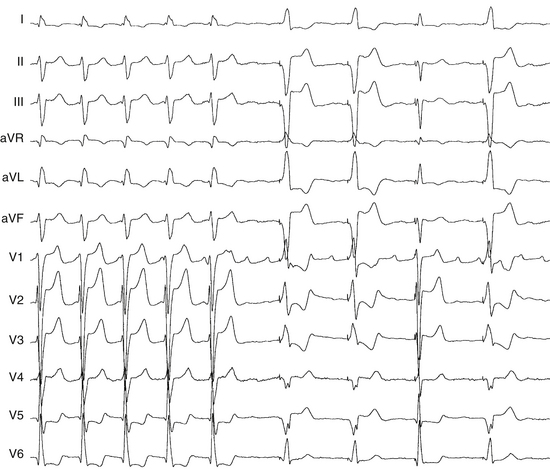
Occasionally, P wave morphology on the surface ECG resembles AFL, but intracardiac recordings show that parts of the atria (commonly the LA) have disorganized atrial activity (Fig. 13-4). Such rhythms behave more like AF than AT, but they may be converted to true typical AFL with antiarrhythmic drugs.
Diagnostic Maneuvers during Tachycardia
Atrial Extrastimulation
A focal mechanism is defined on the basis of dissociation of almost the entire atria from the tachycardia with AES. In contrast, the MRAT circuit usually incorporates large portions of the RA or LA, as demonstrated by resetting. In response to AES, MRATs typically demonstrate an increasing or mixed (flat, then increasing) resetting response. AES usually fails in terminating the AT. AES at short coupling intervals may result in transformation of the tachycardia into a different AT or AF. To minimize the risk to transform or interrupt tachycardia, atrial stimulation maneuvers during the tachycardia should be used sparingly and only when needed to confirm the diagnosis or more precisely localize the critical portion of the AT circuit as guided by the initial activation mapping.12
Atrial Pacing
Entrainment
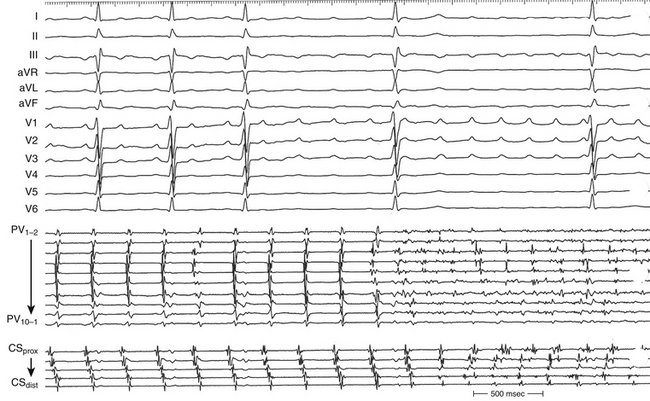
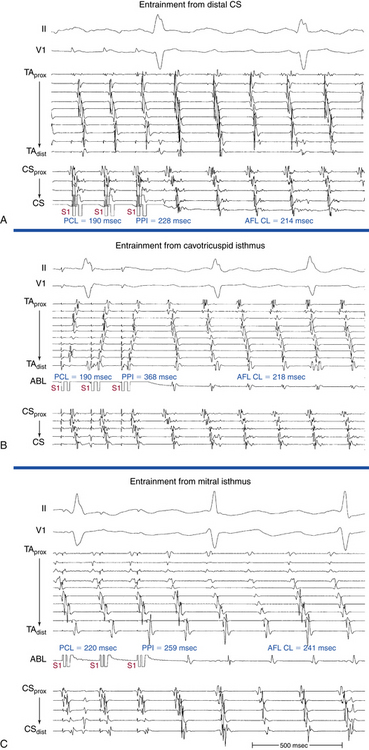
Overdrive atrial pacing at long CLs (i.e., 10 to 30 milliseconds shorter than the tachycardia CL) usually can entrain MRAT. The slower the pacing rate and the farther the pacing site from the reentrant circuit, the longer the pacing drive required to penetrate and entrain the tachycardia. Achievement of entrainment of the AT establishes a reentrant mechanism of the tachycardia and excludes triggered activity and abnormal automaticity as potential mechanisms (Fig. 13-5). Entrainment can also be used to estimate qualitatively how far the reentrant circuit is from the pacing site (see later).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree