Lumbar Disc Arthroplasty
Darren R. Lebl
Federico P. Girardi
Alexander P. Hughes
Frank P. Cammisa Jr.
INDICATIONS
For many years, lumbar spinal fusion has been considered the “gold standard” treatment for degenerative disc disease (DDD) that fails conservative therapy. Reports of symptomatic adjacent segment disease (ASD) at the levels cephalad or caudal to a fusion have been estimated to occur in as many as 16% of patients at 5 years and 36% of patients at 10 years postoperatively (4). As such, lumbar total disc replacement (TDR) has become a widely performed operation in many parts of the world with encouraging early to midterm outcomes (10). FDA approval in 2004 of the Charité (11) (Depuy Spine, Raynham, MA) and in 2006 of the Prodisc-L (12) (Synthes Spine, West Chester, PA) has expanded TDR utilization in the United States for the treatment of one-level DDD from L3-S1 (Prodisc-L) to L4-S1 (Charité). At present, the indications and optimal selection of patients for TDR remain a topic of ongoing debate.
Generally, skeletally mature patients with chronic discogenic low back pain (LBP) due to DDD that is unresponsive to a minimum of 6 months of conservative measures such as physical therapy, weight loss, activity modification, pain management, and injections may be considered for lumbar TDR. Clinical history of symptomatic DDD that is consistent with physical examination and imaging studies is important for appropriate patient selection. A lack of clear consistency between these methods of evaluation may signal involvement of other disease processes considered in the differential diagnosis, psychiatric disorders, secondary gain issues, or narcotic pain medication dependency.
CONTRAINDICATIONS
Active infection, clinically significant facet joint arthropathy, metal (or polyethylene) allergy to the TDR device material, morbid obesity, rheumatologic disorders, clinically significant central or lateral recess stenosis, and greater than grade 1 spondylolisthesis are absolute contraindications to lumbar TDR. Current FDA approval recommends TDR be avoided in patients with objective osteopenia or osteoporosis (T-score less than -1) (12). Greater than 3 mm of anterolisthesis, 11 degrees of scoliosis, bilateral pars defects, and iatrogenic instability following posterior decompression are also contraindications to lumbar TDR (12).
PREOPERATIVE PREPARATION
Preoperative preparation of the candidate for lumbar TDR includes patient education, thorough history and physical examination to rule out any of the above potential contraindications, and study of preoperative radiography. Preoperative CT scan permits analysis of the morphology of the patient’s bony endplates at the planned TDR level for preoperative consideration of device sizing. Variations in sacral morphology or endplate irregularities may preclude stable fixation of the metallic TDR endplate. MRI will help to evaluate any retrovertebral disc material or foraminal stenosis that requires surgical decompression. Concordant pain on provocative discography may provide
additional information to the practitioner to aid in patient selection; however, it has not been shown to be highly predictive of identifying bona fide intradiscal lesions causing chronic LBP (2) and may accelerate disc degeneration at the control levels (1). As such, the authors do not recommend routine discography as part of the preoperative imaging studies. Preoperative lumbar spine MRI and CT may be reviewed to determine the level of aortic and iliac bifurcation and to screen for the presence of any vascular anomalies that may complicate the exposure.
additional information to the practitioner to aid in patient selection; however, it has not been shown to be highly predictive of identifying bona fide intradiscal lesions causing chronic LBP (2) and may accelerate disc degeneration at the control levels (1). As such, the authors do not recommend routine discography as part of the preoperative imaging studies. Preoperative lumbar spine MRI and CT may be reviewed to determine the level of aortic and iliac bifurcation and to screen for the presence of any vascular anomalies that may complicate the exposure.
TECHNIQUE
Patient Positioning and Setup
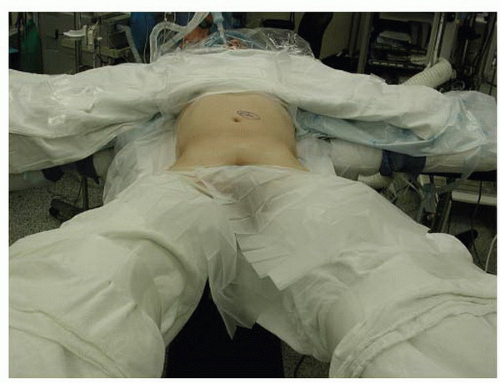
Following the induction of general endotracheal anesthesia, the patient is positioned in the “da Vinci position”—supine with the arms abducted 90 degrees and the legs abducted (Fig. 28-1). Patient positioning should allow for C-arm positioning circumferentially around the operative table. A lateral fluoroscopic image taken prior to prepping and draping with a radiographic marker at the site of the planned skin incision will ensure the exposure is at the appropriate level (Figs. 28-2 and 28-3). Anatomic variations in the patient’s sacropelvic anatomy (pelvic incidence = pelvic tilt + sacral slope) may preclude safe surgical approach to the operative disc space. The abdominal region is prepped and draped from the xiphoid process to the symphysis pubis and laterally to the anterior axillary line.
Surgical Exposure
Routine anterior retroperitoneal approach to the lumbar spine is performed by 4 to 6 cm left-sided paramedian transverse skin incision for single-level cases. A right-sided approach may alternatively be performed in the setting of prior abdominal surgery. Many spine surgeons perform this approach assisted by a vascular access surgeon. The external oblique fascia is identified and is incised just to the left of midline to allow blunt finger elevation and mobilization of the rectus sheath laterally or toward the midline (Fig. 28-4A and B). The posterior rectus sheath is incised longitudinally, and the peritoneal contents are gently swept medially by blunt manual dissection. The peritoneum is elevated away from the psoas muscle with care that the ureter remains medial along with the peritoneal contents. Iliac artery pulsations may be palpated, and the L5-S1 disc space can be exposed in the interval between the bifurcated iliac vessels. The middle sacral vessels may course through the midline on the anterior aspect of the L5-S1 disc space and require ligation. Gentle retraction of the iliac vessels laterally and superiorly is necessary for adequate exposure of L5-S1.
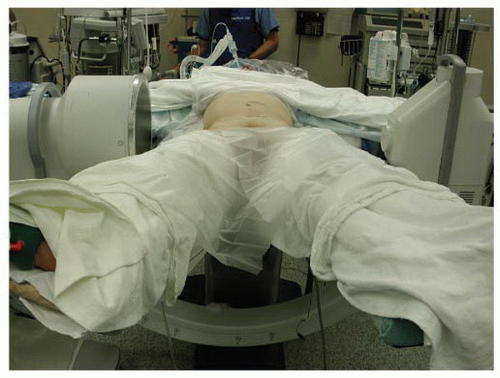 FIGURE 28-2 Lateral fluoroscopic measurement is essential for proper exposure of the operative segment TDR balancing intraoperatively. |
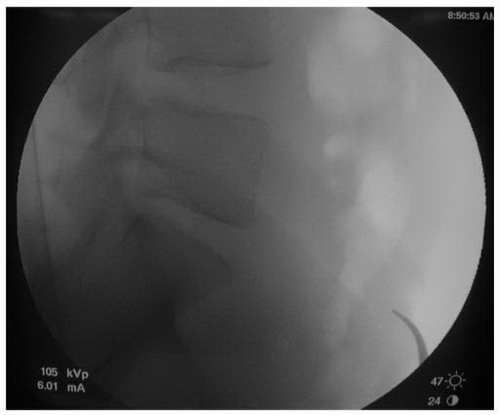 FIGURE 28-3 A radiopaque marker such as a curved hemostat placed over the region of the skin incision will ensure that the patient’s lumbosacral anatomy permits safe and adequate exposure. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree