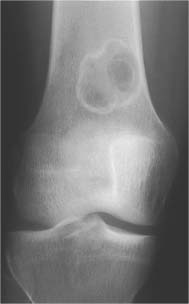
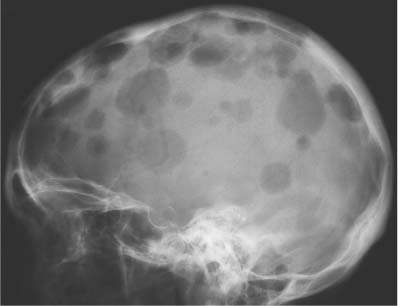
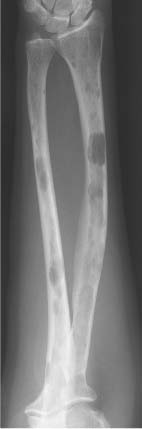
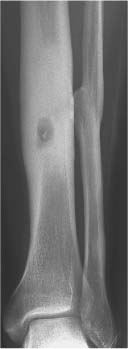
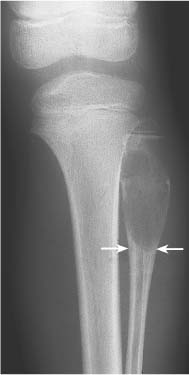
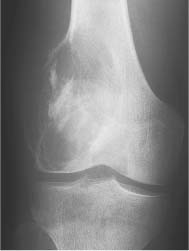
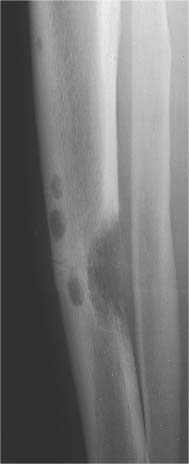
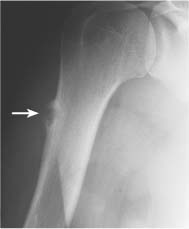
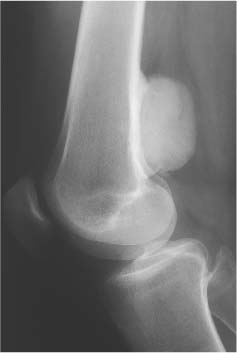
5 Localized Bone Lesions Conventional radiography remains the primary imaging modality for the evaluation of skeletal lesions. The combination of conventional radiography, which has a high specificity but only an intermediate sensitivity, with radionuclide bone scanning, which has a high sensitivity but only a low specificity is still the most effective method for detecting and diagnosing bone lesions and differentiating between benign and malignant conditions. Conventional radiography, is, however, limited in delineating the intramedullary extent of a bone lesion and even more so in demonstrating soft-tissue involvement. Although magnetic resonance imaging frequently contributes to the characterization of a bone lesion, its greatest value lies in the ability to accurately assess the intramedullary and extraosseous extent of a skeletal lesion. A solitary bone lesion is often a tumor or a tumor-like abnormality, but congenital, infectious, ischemic and traumatic disorders can present in similar fashion. Differentiation between a benign or malignant bone lesion is not always possible. Signs of an aggressive or malignant osseous lesion include rapid growth, large size, poor demarcation, cortical violation, interrupted periosteal reaction and soft tissue extension. Signs of a nonaggressive or benign osseous lesion include slow growth, small size, sharp margination, cortical expansion without cortical violation, solid periosteal reaction and no soft tissue extension. However these radiologic features are not infallible and many exceptions occur indicating the need for histologic confirmation in the appropriate setting. In osteolytic lesions a geographic, moth-eaten and permeative pattern of bone destruction are commonly discerned. A geographic lesion (Figs. 5.1 and 5.2) has a well-defined margin separating it clearly from the surrounding normal bone. The zone of transition of normal to abnormal bone is short and a sclerotic border of various thickness may surround the lesion. Geographic lesions are usually benign, especially when they are marginated by a sclerotic rim. Multiple myeloma and metastases, however, frequently present as geographic lesions without sclerotic borders (Table 5.1). A moth-eaten lesion (Fig. 5.3) is a poorly demarcated focus of bone destruction with a long zone of transition from normal to abnormal bone indicating its aggressive nature and rapid growth potential. Malignant bone tumors, osteomyelitis and eosinophilic granulomas frequently present with this pattern of bone destruction (Table 5.2). A permeative lesion (Fig. 5.4) represents the most aggressive bone destruction pattern with rapid growth. The lesion merges imperceptibly with the normal bone. Highly malignant tumors infiltrating the bone marrow such as round cell sarcomas (e.g. Ewing’s sarcomas and lymphomas) typically are associated with this pattern of bone destruction. It is, however, also found in acute osteomyelitis and rapidly developing osteoporosis such as reflex sympathetic dystrophy. Infiltration of the cortex may also be associated with these conditions, presenting as cortical striation or tunneling. Fig. 5.1 Geographic lesion. A well-demarcated lesion with sclerotic border is seen in the distal femur (nonossifying fibroma). Fig. 5.2 Geographic lesions. Multiple well-demarcated (punched-out) purely lytic lesions are seen in the vault of the cranium (multiple myeloma). Fig. 5.3 Moth-eaten lesion. A poorly demarcated osteolytic lesion (arrows) is seen in the distal femur (non-Hodgkin lymphoma). Table 5.1 Solitary well defined osteolytic lesion
Subchondral cyst (associated with arthritis, osteonecrosis, or trauma) |
Gout (intraosseous tophus) |
Amyloidosis |
Intraosseous ganglion |
Simple (unicameral) bone cyst |
Aneurysmal bone cyst |
Epidermoid inclusion cyst |
Glomus tumor |
Intraosseous lipoma |
Enchondroma |
Chondroblastoma |
Chondromyxoid fibroma |
Nonossifying fibroma |
Desmoplastic fibroma |
Osteoblastoma |
Giant cell tumor |
Fibrosarcoma |
Clear cell chondrosarcoma |
Angiosarcoma |
Plasmacytoma/multiple myeloma |
Metastasis |
Eosinophilic granuloma |
Brown tumor (hyperparathyroidism) |
Hemophilic pseudotumor |
Osteonecrosis (bone infarct) |
Brodie’s abscess/cystic osteomyelitis |
Fibrous dysplasia |
Sarcoidosis |
The cortex represents a barrier to nonaggressive lesions. Benign medullary processes may leave the endosteal surface intact or produce endosteal scallopin g (Fig. 5.5). The latter finding is, however, also frequently seen with multiple myeloma and metastases. Progressive endosteal erosion associated with solid new periosteal bone deposition creates an expanded osseous contour indicative of a nonaggressive benign skeletal lesion. Aggressive skeletal lesions may penetrate the entire thickness of the cortex (Fig. 5.6) and sometimes induce a variety of interrupted periosteal reactions including onion-peel, sunburst and hair-on-end patterns or a Codman’s triangle. They are most commonly associated with osteosarcoma, Ewing’s sarcoma, and osteomyelitis and are discussed in greater detail in chapter 3.
Table 5.2 Solitary poorly defined osteolytic lesion
Hemangioma |
Chondroblastoma |
Osteoblastoma |
Giant cell tumor |
Fibrosarcoma |
Malignant fibrous histiocytoma |
Chondrosarcoma |
Osteosarcoma |
Ewing’s sarcoma |
Angiosarcoma |
Multiple myeloma Metastasis |
Lymphoma |
Langerhans cell histiocytosis (eosinophilic granuloma) |
Hemophilic pseudotumor |
Osteonecrosis (bone infarct) |
Osteomyelitis |
Brodie’s abscess |
Sarcoidosis |
Fig. 5.4 Permeative lesion. A poorly defined osteolytic lesion merging imperceptibly with the normal bone is seen in the proximal femur. Note also the beginning laminated periosteal reaction in the subtrochanteric area (Ewing sarcoma).
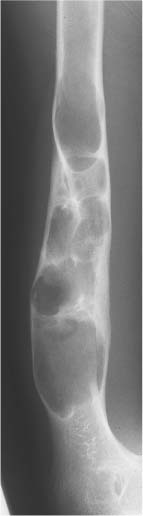
Fig. 5.5 Endosteal scalloping. Sharply demarcated erosions of the inner cortex of the radius and ulna caused by multiple osteolytic lesions is seen (multiple myeloma).
The matrix of a skeletal lesion may be inhomogeneous because it contains areas of calcification or ossification. Calcifications appear as ring-like, flocculent or fleck-like radiodense areas (Figs. 5.7 and 5.8). Intramedullary matrix calcification is primarily associated with cartilaginous tumors and bone infarcts (Table 5.3). Foci of intramedullary ossifications are more homogeneous and often ivory-like and are most often caused by bone islands, osteoblastic metastases and primary bone forming neoplasms (Fig. 5.9). They are discussed in detail in chapter 2.
Fig. 5.6 Cortical penetration. A poorly defined, mixed osteolytic and osteoblastic lesion is seen in the distal femur penetrating through the medial cortex. The lateral cortex is expanded and thinned but still intact (osteosarcoma).
Table 5.3 Bone lesions with calcification
Intraosseous lipoma |
Osteochondroma |
Enchondroma |
Periosteal (juxtacortical) chondroma |
Bizarre parosteal osteochondromatous proliferation (BPOP) |
Chondroblastoma |
Dysplasia epiphysealis hemimelica (Trevor’s disease) |
Fibrocartilagenous mesenchymoma |
Chondromyxoid fibroma |
Osteoid osteoma (nidus) |
Osteoblastoma (nidus) |
Ossifying fibroma |
Gnathic tumors (see Chapter 11) |
Chordoma |
Chondrosarcoma (all variants) |
Metastasis (especially thyroid carcinoma) |
Gout (intraosseous tophus) |
Osteonecrosis (bone infarct) |
Intraosseous hematoma |
Osteogenesis imperfecta (popcorn calcifications in enlarged epimetaphyses) |
Fig. 5.7 Matrix calcification. A flocculent, ring-like cluster of calcification is seen in the distal femur (enchondroma).
Fig. 5.8 Matrix calcification. An irregular, shell-like calcification is seen in the distal femur (bone infarct).
Fig. 5.9 Intramedullary ossification. An irregular, ivory-like area of sclerosis is seen in the proximal humerus (enostosis or giant bone island).
Fig. 5.10 Septation. A lytic lesion with extensive delicate trabeculation induced by the tumor is seen in the iliac wing. Note also the localized cortical violation (arrow) in the superolateral aspect of the lesion (aneurysmal bone cyst).
Septation of the matrix represents another mechanism of new bone formation evoked by a neoplasm (Fig. 5.10). In other instances intratumoral septations represent the remnants of the original bone matrix largely destroyed by the neoplasm (Fig. 5.11). Septation is associated with both benign and malignant lesions. Delicate thin trabeculae typically are found in giant cell tumors and aneurysmal bone cysts, lobulated trabeculae in nonossifying fibromas, spiculated or radiating trabeculae in hemangiomas and irregular coarse trabecula in a variety of benign and malignant lesions, often of fibrous connective tissue origin (Table 5.4). A uniform hazy increase in radiodensity in an osteolytic lesion is termed ground glass appearance. It is most characteristic for fibrous dysplasia (Fig. 5.12), but is occasionally also found in simple (unicameral) bone cysts in the adult. The demonstration of a sequestrum (Fig. 5.13) representing a segment of dense necrotic bone is indicative of chronic osteomyelitis (Table 5.5).
Table 5.4 Osteolytic lesions with trabeculation/septation
Simple (unicameral) bone cyst |
Aneurysmal bone cyst |
Intraosseous lipoma |
Hemangioma |
Chondromyxoid fibroma |
Nonossifying fibroma |
Ossifying fibroma |
Giant cell tumor |
Gnathic tumors (see Chapter 11) |
Adamantinoma |
Ameloblastoma |
Fibrosarcoma |
Malignant fibrous histiocytoma |
Osteosarcoma, teleangiectatic |
Plasmacytoma/multiple myeloma |
Metastasis (e.g. blowout-metastases from kidney, thyroid orlung) |
Brown tumor (hyperparathyroidism) |
Hemophilic pseudotumor |
Fibrous dysplasia |
Sarcoidosis |
Fig. 5.11 Septation. A large expansile lytic lesion with remaining remnants of the original bone matrix producing a septated appearance, is seen in the ilium (plasmacytoma).
Fig. 5.12 Ground glass appearance. A slightly expansile osteolytic lesion with a hazy increase in density is seen in the proximal tibia (fibrous dysplasia).
Fig. 5.13 Bone sequestrum. A lytic lesion containing a small sclerotic sequestrum in its center is surrounded by dense sclerosis and cortical thickening in the tibia (chronic osteomyelitis). A healed fibula fracture is incidentally also seen.
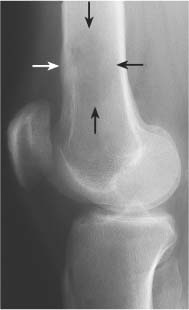
Bone expansion can be associated with both benign and malignant lesions. In a slowly growing tumor the bone erosion on the inner cortex is compensated by bone apposition on the outer cortex (Fig. 5.14). In this instance the cortex remains intact at all times, but the thickness of this new cortical shell may be different when compared to the original cortex. The interface between normal and expanded cortex may be filled in with dense bone and often is referred to as buttressing (Fig. 5.15). It is found, among others, with eosinophilic granulomas, aneurysmal bone cysts and osteoblastomas. In rapidly growing tumors the new bone formation cannot keep up with the bone breakdown resulting in cortical violation or frank destruction (Fig. 5.16) (Tables 5.6 and 5.7).
Fig. 5.14 Expansile lesion with intact cortex. An expansile, multiloculated lesion with intact, thinned or thickened cortex is seen in the distal humerus (simple [unicameral] bone cyst).
Table 5.5 Osteolytic lesions containing a sequestrum or bone fragment
Simple (unicameral) bone cyst with pathologic fracture (fallen fragment sign) |
Fibrosarcoma (sequestered bone fragment) |
Metastasis (sequestered bone fragment) |
Bone lesion with pathologic fracture (fracture fragment) |
Eosinophilic granuloma (sequestrum) |
Comminuted fracture (intramedullary displaced cortical fragment) |
Osteomyelitis (sequestrum) |
Brodie’s abscess (sequestrum) |
Infected pin tract (ring sequestrum) |
Button sequestrum in skull (eosinophilic granuloma, metastases, epidermoid, osteoblastoma, osteomyelitis, radiation necrosis, bone flap undergoing avascular necrosis, burr hole and normal variants) |
Table 5.6 Expansile osteolytic lesion with intact cortex
Simple (unicameral) bone cyst |
Aneurysmal bone cyst (eccentric) |
Enchondroma |
Chondromyxoid fibroma (eccentric) |
Nonossifying fibroma (eccentric) |
Desmoplastic fibroma |
Osteoblastoma |
Giant cell tumor (eccentric) |
Fibrosarcoma |
Chondrosarcoma |
Eosinophilic granuloma |
Brown tumor (hyperparathyroidism) |
Hemophilic pseudotumor |
Healing/healed fracture |
Osteomyelitis (e.g. spina ventosa [phalanges or metacarpals] in tuberculosis) |
Fibrous dysplasia |
Fig. 5.15 Buttressing. Localized cortical thickening (arrows) is seen in the proximal fibula at the interface between normal cortex and expanded cortex of the osteolytic lesion (aneurysmal bone cyst).
Fig. 5.16 Cortical destruction. An eccentric, expansile, osteolytic lesion in the distal femur metaphysis and epiphyses broke through the cortex with only a few cortical remnants remaining (giant cell tumor).
The location of a solitary lesion within a bone provides an important clue to the correct diagnosis. In tubular bones the epiphysis is a common location for chondroblastomas, clear cell chondrosarcomas, metastases, lipomas, subchondral cysts, intraosseous ganglia and Brodie’s abscesses. Giant cell tumors originate in the metaphysis, but quickly penetrate the closed growth plate and extend into the subchondral bone. Osteoid osteomas (intra-articular presentation), enchondromas and eosinophilic granulomas occasionally are also found in the epiphyses, but the diametaphysis is a more characteristic location for these tumors (Table 5.8). Lesions commonly located in the epiphyses are also found about the joints of flat bones, patella and carpal and tarsal bones.
Typical metaphyseal lesions include nonossifying fibroma which characteristically develops a short distance from the growth plate, chondromyxoid fibroma which abuts the growth plate, simple (unicameral) bone cyst, aneurysmal bone cyst, osteochondroma, Brodie’s abscess, mesenchymal sarcomas such as osteosarcoma and chondrosarcoma and metastases. Common diaphyseal lesions include round cell tumors (e.g. Ewing’s sarcoma and lymphoma), metastases, nonossifying fibromas, simple (unicameral) bone cysts in adults, enchondromas, osteoid osteomas, osteoblastomas, and fibrous dysplasia.
The diagnosis of a tubular bone lesion is also facilitated by the identification of its center with regard to the medullary canal and cortex. Typical central lesions include simple (unicameral) bone cysts, enchondromas, fibrous dysplasia and bone infarcts. Eccentric lesions include aneurysmal bone cysts, giant cell tumors and chondromyxoid fibromas. Typical cortical lesions are nonossifying fibromas and osteoid osteomas (Fig. 5.17). Surface lesions arise from the outer surface of the cortex (e.g. surface high-grade osteosarcoma). Juxtacortical lesions (Figs. 5.18 and 5.19) can be divided into those originating from the deep layer of the periosteum (periosteal lesions) and those derived from the outer layer of the periosteum and growing in an exophytic pattern (parosteal lesions). Typical examples of juxtacortical lesions include the periosteal osteosarcoma and parosteal osteosarcoma (Table 5.9).
Table 5.7 Expansile osteolytic lesion with cortical violation
Aneurysmal bone cyst (eccentric) |
Epidermoid inclusion cyst |
Glomus tumor |
Hemangioma (skull) |
Chondromyxoid fibroma (eccentric) |
Desmoplastic fibroma |
Osteoblastoma |
Giant cell tumor (eccentric) |
Fibrosarcoma |
Malignant fibrous histiocytoma |
Chondrosarcoma |
Osteosarcoma |
Angiosarcoma |
Plasmacytoma/multiple myeloma |
Metastases (from kidney, thyroid, lung) |
Hemophilic pseudotumor |
Table 5.8 Common location of tubular bone lesions
Epiphyses |
Subchondral cyst (associated with arthritis, osteonecrosis or trauma) |
Gout (intraosseous tophus) |
Amyloidosis |
Intraosseous ganglion |
Intraosseous lipoma |
Chondroblastoma |
Dysplasia epiphysealis hemimelica (Trevor’s disease) |
Giant cell tumor (originates in metaphysis) |
Clear cell chondrosarcoma |
Metastasis |
Brodie’s abscess |
Metaphyses |
Simple (unicameral) bone cyst |
Aneurysmal bone cyst |
Osteochondroma |
Chondromyxoid fibroma |
Periosteal desmoid |
Nonossifying fibroma |
Desmoplastic fibroma |
Fibrosarcoma |
Malignant fibrous histiocytoma |
Chondrosarcoma |
Osteosarcoma |
Metastasis |
Osteomyelitis |
Brodie’s abscess |
Fibrous dysplasia |
Diametaphysis |
Simple (unicameral) bone cyst (in adults) |
Intraosseous lipoma |
Enchondroma |
Periosteal chondroma |
Nonossifying fibroma |
Bone island |
Osteoid osteoma |
Osteoblastoma |
Adamantinoma (especially anterior tibia) |
Fibrosarcoma |
Malignant fibrous histiocytoma |
Ewing’s sarcoma |
Angiosarcoma |
Multiple myeloma |
Metastasis |
Lymphoma |
Langerhans cell histiocytosis (eosinophilic granuloma) |
Brown tumor (hyperparathyroidism) |
Hemophilic pseudotumor |
Osteonecrosis (bone infarct) |
Osteomyelitis |
Fibrous dysplasia |
Osteofibrous dysplasia (especially anterior tibia) |
The differential diagnosis of localized bone lesions is discussed in Table 5.10.
Localized elliptical cortical thickening |
Osteoid osteoma |
Osteomyelitis (chronic) |
Stress fracture |
Localized defect of external cortex |
Periosteal desmoid (posteromedial aspect of distal femur) |
Fibromatosis |
Adamantinoma (especially anterior tibia) |
Osteofibrous dysplasia (especially anterior tibia) |
Ewing’s sarcoma |
Metastasis (especially from bronchogenic carcinoma) |
Hyperparathyroidism including brown tumor |
Gouty tophus |
Adjacent soft tissue neoplasm or abscess |
Subperiosteal osteomyelitis |
Subperiosteal hematoma |
Avulsion fracture/injury (chronic) |
Juxtacortical tumors and tumor-like lesions |
Parosteal lipoma (may induce cortical thickening or hyperostosis |
Parosteal hemangioma (may induce cortical thickening or hyperostosis |
Osteoma |
Osteochondroma |
Periosteal (juxtacortical) chondroma |
Bizarre parosteal osteochondromatous proliferation (BPOP) |
Periosteal (juxtacortical) desmoid |
Peripheral chondrosarcoma |
Periosteal chondrosarcoma |
Periosteal osteosarcoma |
Parosteal osteosarcoma |
Parosteal myositis ossificans |
Spurs (congenital, post-traumatic) |
Enthesopathy |
Osteophytosis |
Fig. 5.17 Cortical lesions. Multiple osteolytic lesions are seen within the cortex of the tibia. The largest lesion has extended from the cortex into the medullary space and adjacent soft tissue (metastases from bronchogenic carcinoma).
Fig. 5.18 Juxtacortical lesion. A defect (arrow) with minimal punctate matrix calcifications is seen in the outer cortex of the proximal humerus surrounded by periosteal new bone formation (periosteal [juxtacortical] chondroma).
Fig. 5.19 Juxtacortical lesion. An ossified mass with adjacent cortical thickening is seen in the posterior aspect of the distal femur (parosteal osteosarcoma).
Table 5.10 Localized bone lesions
Disease | Radiographic Findings | Comments |
Subchondral cyst (Fig. 5.20) | Solitary or multiple defects measuring up to 3 cm in diameter with sclerotic border located in the subchondral bone (pressure segment) of a joint. Communication with joint may be present occasionally allowing gas to pass into the cyst (pneumatocyst). | Associated with osteoarthritis, calcium pyrophosphate dihydrate crystal deposition disease (CPPD), osteonecrosis (avascular necrosis) and rheumatoid arthritis). Traumatic cysts are the sequela of a bone injury, but may be larger and not necessarily associated with an arthritic process. |
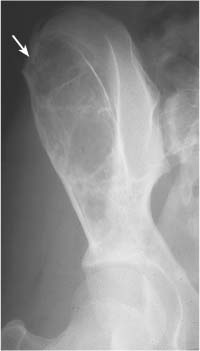
Intraosseous ganglion (Fig. 5.21) | Unilocular or multilocular, well-defined lytic epiphyseal lesion, often with sclerotic margin. The lesion does not communicate with the adjacent joint. Preferred locations are the medial malleolus of the tibia, femoral head, acetabulum and carpal bones. | A herniation pit (Fig. 5.22) is a well-circumscribed radiolucent defect usually measuring less than 1 cm in diameter with a thin sclerotic border in the superolateral aspect of the femoral neck caused by herniated synovium. |
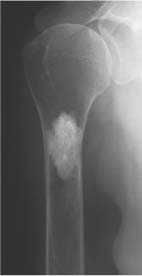
Simple (uni-cameral) bone cyst (Figs. 5.23, 5.24 and 5.25) | Centrally located, well-demarcated and often slightly expansile osteolytic lesion with cortical thinning is characteristic. The lesion may be septated or depict a scalloped inner margin. Most often located in long tubular bones (especially humerus and femur), initially in the metaphysis abutting the growth plate and with time migrating into the diaphysis. Pathologic fracture is relatively common with a fracture fragment occasionally located at the bottom of the cyst (fallen fragmentsign). | Asymptomatic unless fractured. Usually diagnosed before the age of 20 years with a 3:1 male predominance. After that age bone cysts tend to appear more often in flat bones such as pelvis or calcaneus. Echinococcal (hydatid) cysts are found in 1% of infected patients presenting with single or multiple expansile cystic lesions (“bunch of grapes”) preferentially located in the pelvis and sacrum. |
Aneurysmal bone cyst (Figs. 5.26 and 5.27) | Presents most commonly as an eccentric, expansile osteolytic lesion with intact or disrupted cortex in the metaphysis of a long tubular bone. Extensive sclerosis at the interface between normal and expanded cortex (“buttressing”) may be present. Delicate thin trabeculation is characteristic and an expansile ballooning lesion may produce a “soap-bubble” appearance. Other common locations include the posterior elements of the spine and the pelvis, where a large soft tissue component may simulate a malignancy. | Primary lesions are usually diagnosed in the first, second or less frequently third decades of life with slight female predominance. Secondary aneurysmal bone cysts (20%) are found in giant cell tumors and less frequently in osteoblastomas, simple bone cysts, nonossifying fibromas, teleangiectatic osteosarcomas, metastases, fibrous dysplasia, Paget’s disease, and others. |
Epidermoid (inclusion cyst) Figs. 5.28 and 5.29) | Intraosseous lesion presents as well-defined osteolytic defect, often with sclerotic margin, occurring in the terminal phalanx of the hand or in the skull. The lesion may be expansile with thinning or destruction of the cortex. Skull lesions usually measure 1 to 5 cm in diameter and may be limited to the diploic space or extend through one or both tables. An irregularly shaped button sequestrum is usually present in larger skull lesions. | Usually diagnosed in the second to fourth decades of life with slight male predominance. A history of trauma is often present suggesting intraosseous implantation of ectodermal tissue with subsequent development of an epidermoid. The lesion is lined with a stratified squamous epithelium shedding keratin debris that breaks down and forms cholesterol. |
Glomus tumor | Intraosseous glomus tumor presents as a well-defined osteolytic lesion in the terminal phalanges of the hand indistinguishable from an epidermoid on plain film radiography. Differentiation is however possible with MR imaging, where the glomus tumor depicts marked contrast enhancement, often with characteristic “salt and pepper” appearance. | Glomus tumors occur in patients of any age, typically are neither palpable nor visible and present in fingertips with aching pain and point tenderness. Secondary bone involvement from a soft tissue glomus tumor is much more common (e.g. temporal bone). In the hand such a lesion presents as shallow, well-marginated erosion in the adjacent bone, usually in the tuft of a terminal phalanx. |
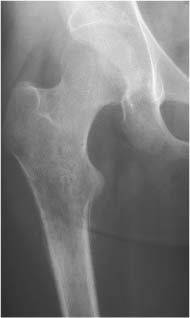
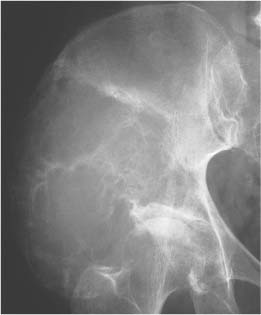
Lipoma (Fig. 5.30) | Well-circumscribed osteolytic lesion, often surrounded by a sclerotic border. Irregular thick bony ridges are frequently found in larger lesions. May contain a central calcified nidus (especially in the calcaneus). Demonstration of fatty tissue within the lesion by either CT or MR imaging is virtually diagnostic. | Besides the calcaneus intraosseous lipoma occur most commonly in the metaphyses of long tubular bones, especially the femur, tibia and fibula. A relatively typical location of larger lipomas is the femoral neck abutting the intertrochanteric line. In this location they resemble fibrous dysplasia. Liposarcomas rarely arise in bone. |
Liposclerosing myxofibroma (Liposclerosing myxofibrous tumor, LSFMT) (Fig. 5.31) | Well defined lytic or “ground glass” lesion, often with markedly sclerotic-border occurring typically (80%) in the pretrochanteric and intertrochanteric region of the proximal femur. Fat is not always demonstrated with either CT or MRI. | Rare benign lesion commonly diagnosed as incidental finding or presenting with pain in patients between 20 and 70 years of age. Malignant transformation occurs in less than 10% of cases. May be a variant of fibrous dysplasia or represent a burned-out or infarcted intraosseous lipoma. |
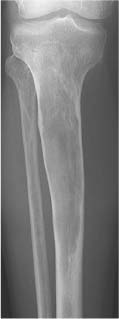
Hemangioma (Figs. 5.32, 5.33 and 5.34) | Spine: Coarse, vertical trabecular pattern (“corduroy” appearance) in the vertebral bodies is characteristic. Diameter of thickened vertical trabeculae is rather uniform (DD: Multiple myeloma, metastases and Paget’s disease, which may present with irregular coarse trabecular thickening). Extension into the posterior elements, paraspinal tissues, and spinal canal is rare. Skull: Slightly expansile osteolytic lesion with radiating (“sunburst”) pattern. Tubular bones: Poorly defined osteolytic lesion with lattice-like trabecular pattern. | Usually found in patients over 40 years of age with female predominance. Occasionally non-Hodgkin’s lymphoma (especially the histiocytic type) and metastases (e.g. breast carcinoma) may simulate a hemangioma in the spine. Cystic angiomatosis (Fig. 5.35) is characterized by widespread cystic lesions often surrounded by a rim of sclerosis. They may progress to osteosclerotic lesions simulating metastases. The condition is frequently associated with widespread visceral involvement. Cystic lymphangiomatosis presents in a similar fashion. Accumulation of contrast material in lesions during lymphography is diagnostic. |
Osteochondroma (Figs. 5.36 and 5.37) | Bony protuberance demonstrating cortical and medullary continuity with parent bone is diagnostic in the tubular bones. The lesion may be pedunculated (with narrow stalk and bulbous tip) or sessile (with a broad, flat base). Osteochondromas characteristically originate from the metaphyses and point away from the nearby articulation. The tip of the osteochondroma is covered by a hyaline cartilage cap that may contain regular stippled calcifications. A large cartilaginous cap containing irregular calcifications is suspicious of malignant transformation. Osteochondromas in the pelvis frequently are large and difficult to differentiate from lesions that have undergone malignant transformation. In the ribs osteochondromas frequently occur at the costochondral junction. Malignant transformation may be best assessed with MR imaging. A cartilage cap of 1 cm or less in thickness indicates a benign lesion, whereas a cap thicker then 2 cm is suspicious for malignant transformation. | Osteochondromas occur in children and adolescents as a slowly growing painless mass. They are intimately related to the physis and cease to enlarge with fusion of the adjacent growth plates. Rarely osteochondromas may develop in the adult after trauma. DD: 1. Subungual exostosis (Fig. 5.38) projects from the dorsomedial aspect of the tuft, usually of the great toe (80%) or other distal phalanges of the foot or hand. 2. Reactive bony excrescence in medial portion of base of distal phalanx of great toe (normal variant). 3. Turret exostosis arises on dorsal surface of proximal or middle phalanx in a finger (post-traumatic). 4. Supracondylar process of humerus (Fig. 5.39): Spur originating from the anteromedial aspect of the distal humerus pointing towards the elbow (phylogenetic vestige). 5. Pes anserinus spur may mimic a small osteochondroma in the medial aspect of the proximal tibia (enthesophyte, occasionally associated with anserinus bursitis). 6. Post-traumatic spurs secondary to healed avulsion fractures. 7. “Tug” lesions at tendinous attachment sites in the lower extremity. Hereditary multiple exostoses (Fig. 5.40) present with multiple, usually bilateral and symmetric osteochondromas in the axial and appendicular skeleton associated with bone modeling deformities. In metachondromatosis (Fig. 5.41) multiple osteochondromas and enchondromas coexist, especially in the hands and feet. |
Dysplasia epiphysealis hemimelica (Trevor’s disease) (Fig. 5.42) | Lobulated osseous mass (articular chondroma) protruding from an epiphysis, carpal or tarsal bone. Presents initially in infants as irregular ossifications adjacent to the involved bone. Preferential involvement of the medial side of a lower extremity (e.g. distal femur, proximal and distal tibia and talus). Multiple bones in a single extremity are affected in two-thirds of cases. | Presents in children and young adults with swelling, pain and deformity localized to one side of the body. |
Enchondroma (Figs. 5.43 and 5.44) | Well-circumscribed, often lobulated, osteolytic lesion with endosteal scalloping composed of hyaline-type cartilage with varying degrees of calcifications. Preferred locations are the metaphyses of the long tubular bones and the diaphyses in the short tubular bones of the hands and feet. Cortical expansion and pathologic fractures are frequent findings in the hands and feet, but malignant transformation is very rare in these locations. Transformation to chondrosarcoma in the long tubular and flat bones should be suspected with an enlarging radiolucent area, the disappearance of pre-existing calcifications or pathologic fracture. | Enchondromas are usually discovered in the third and fourth decades of life as incidental findings. The presence of pain should arouse suspicion of malignant transformation. Low-grade chondrosarcomas in the long tubular bones and axial skeleton are difficult if not impossible to differentiate from benign enchondromas. Enchondromatosis (Ollier’s disease) (Figs. 5.45 and 5.46) is characterized by multiple, asymmetrically distributed enchondromas, either exclusively or predominantly involving one side of the body. The affected bones often are shortened and deformed. After cessation of growth the lesion no longer increases in size unless malignant transformation has taken place, estimated to occur in 5 to 30% of patients. Maffucci’s syndrome (Fig. 5.47) consists of multiple enchondromas and soft tissue hemangiomas containing multiple phleboliths. |
Periosteal (juxtacortical) chondroma (Figs. 5.48 and 5.49) | Calcified (50%) soft tissue mass with erosion of the adjacent cortex and varying degree of periostitis. Metaphyses of long bones and hands are most commonly affected. | All ages are affected but the tumor is usually diagnosed under age 30. Slight male predominance. |
Bizarre parosteal osteochondromatous proliferation (BPOP) (Fig. 5.50) | Well-marginated sessile or pedunculated mass of heterotopic ossification arising from the cortical surface without medullary continuity between the lesion and the adjacent bone is characteristic. | Occurs usually in the hands and feet (occasionally in long tubular bones) without age and sex predilection. A history of trauma is evident in some patients. |
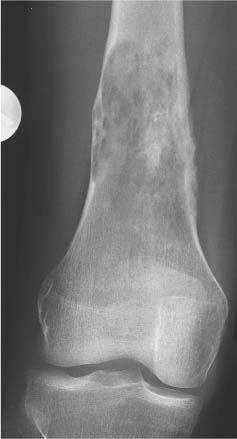
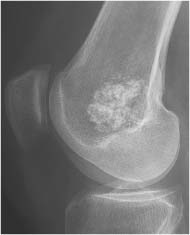
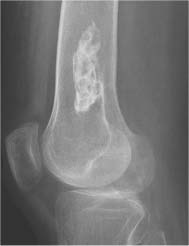
Chondroblastoma (Figs. 5.51 and 5.52) | Well-defined, often lobulated lytic lesion involving the epiphysis or apophysis of a long tubular bone with or without thin sclerotic border and matrix calcification (> 50%) is characteristic. A solid periosteal reaction in the adjacent metaphyseal shaft can be induced by bone marrow edema surrounding the lesion. Approximately 10% of chondroblastomas occur in the hands and feet with predilection for the talus and calcaneus. | Benign cartilaginous lesion occurring between the ages of 5 and 25 years with slight male predominance. |
Chondromyxoid fibroma (Fig. 5.53) | Eccentric metaphyseal osteolytic lesion with cortical expansion, coarse trabeculation, endosteal scalloping and scalloped sclerotic medullary border is commonly seen. Destruction of the cortex resulting in a hemispherical osseous defect or “bite” without periosteal reaction is characteristic of larger lesions. Predilection for the long tubular bones of the lower extremity, but occasionally also found in pelvis and foot. | Uncommon benign cartilaginous lesion occurring between the ages of 5 and 25 years with slight male predominance and presenting with slowly progressive pain, tenderness, swelling and restriction of motion is typical. Fibrocartilagenous mesenchymoma is a very rare solitary expansile osteolytic lesion with spotty or ring-like calcifications, cortical destruction and soft tissue invasion originating most often in the metaphysis of long tubular bones. Age distribution is similar to chondromyxoid fibroma. |
Periosteal (juxtacortical) desmoid (Fig. 5.54) | Saucer-like defect in the posteromedial cortex of the distal femur often associated with adjacent sclerosis, periostitis and soft-tissue swelling. | Occurs between the age of 10 and 20 as sequelae of acute or chronic trauma at the adductor magnus tendon insertion in the linea aspera. |
Nonossifying fibroma (Figs. 5.55, 5.56 and 5.57) | Small lesions present as round or oblong, well delineated, radiolucent areas in the cortex with normal or sclerotic adjacent bone. They typically arise in the metaphysis at a short distance from the physis. Larger lesions present as a well delineated, eccentric, ovoid osteolytic area in the diametaphyses. They frequently have a multiloculated appearance and both cortical expansion and thinning may be evident. The long tubular bones, especially the tibia and femur, are most frequently affected. With time the lesions may spontaneously disappear or become sclerotic. | Usually diagnosed in patients under the age of 20. Smaller lesions are referred to as benign fibrous cortical defects. They are asymptomatic and diagnosed as incidental finding on routine radiography and occasionally are multifocal. They usually regress spontaneously or less commonly enlarge and migrate with growth into the diaphyses eventually being referred to as nonossifying fibromas. Pathologic fractures in larger lesions are not uncommon. Jaffe-Campanacci syndrome consists of multiple nonossifying fibromas associated with café-au-lait spots, mental retardation and hypogonadism. Benign fibrous histiocytomas (Fig. 5.58) are histologically identical to nonossifying fibromas, but present as slightly more aggressive lesions in patients over age 20 without site predilection. |
Desmoplastic fibroma (Fig. 5.59) | Central osteolytic lesion with trabeculated, soap bubble or honeycomb pattern in the metaphyses of long tubular bones, mandible or pelvis. Slight bony expansion with endosteal erosion and limited periosteal bone formation may be associated. | Rare benign neoplasm occurring in the second or third decades of life. Pain and swelling are the leading clinical symptoms or a pathologic fracture may be the presenting feature. |
Bone island (enostosis) (Figs. 5.60 Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|