(5)
Labrum is University of Pittsburgh Medical School, Arner is Orthopedic Residency UPMC, University of Pittsburgh Medical Center, 3200 South Water Street, Pittsburgh, PA 15203, USA
Take-Home Message
ACL
Two functional bundles named for their insertion sites, the anteromedial (AM) bundle and the posterolateral (PL) bundle (Figs. 1 and 2).
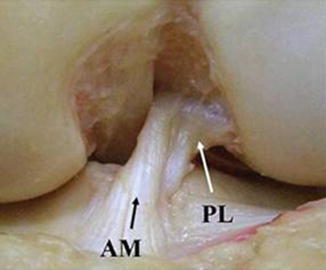
Fig. 1
Cadaveric dissection of human knee delineating the two-bundle composition of the ACL: anteromedial (AM) and posterolateral (PL) bundles (figure courtesy of Dr. Freddie Fu)
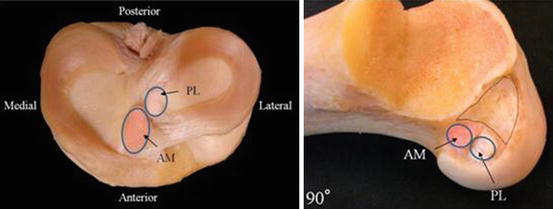
Fig. 2
Cadaveric dissection of human knee depicting the anatomic attachments of the ACL bundles on the tibia and the femur with the knee in 90° of flexion (Courtesy of Dr. Freddie Fu)
The natural history of the ACL-deficient knee in young and active patients may be worsening of meniscal pathology, chondral injury, and early arthritic progression.
No upper age limit exists for reconstruction, should be based on symptoms and activity.
Females have increased risk for ACL rupture.
The MCL and lateral meniscus are common concomitant injuries in acute ACL tears; medial meniscus injuries are seen more in chronic tears.
Many ACL graft options exist; hamstring and bone-tendon-bone grafts both have shown good outcomes.
PCL
Three bundles exist: anterolateral, posteromedial, and meniscofemoral ligaments.
The PCL and PLC work synergistically.
Evaluation of the PLC, LCL, and PCL is important.
PCL has some healing capacity.
Single bundle, double bundle, tibial and transtibial inlay, and PCL augmentation are all options.
Definitions
Anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL) are important knee stabilizers, can be injured to different degrees, and may lead to knee instability and possibly further knee injury.
Anatomy is essential; anatomic reconstruction has shown to improve outcomes.
Anatomy
ACL
Origin on the medial wall of the lateral femoral condyle, insertion on the tibial articular surface.
Two functional bundles named for their insertion sites, the anteromedial (AM) bundle and the posterolateral (PL) bundle (Figs. 1 and 2):
AM bundle is tight in flexion.
PL bundle is tight in extension (Fig. 3).
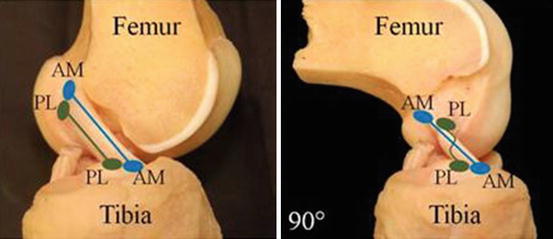
Fig. 3
Schematic showing that when the knee is in extension, the two bundles are parallel to each other. With the knee bent at 90°, the AM bundle is tight and the PL bundle is loose. This allows the knee to remain stable in the anterior direction but also concomitantly allow rotation of the tibiofemoral articulation. The two bundles work synergistically to allow for knee function (Courtesy of Dr. Freddie Fu)
90 % type I and 10 % type III collagen.
Innervation via the posterior articular nerve, a branch of the tibial nerve.
Provides anteroposterior stability and is a secondary rotatory stabilizer.
Blood supply is primarily via the middle genicular artery, a branch of the popliteal artery.
PCL
Technically is extra-articular; synovium reflects around the PCL from the posterior capsule.
Blood supply from the synovium plus same vascular supply (middle genicular artery) and innervation (posterior articular nerve) as the ACL.
Made up of the larger and stronger anterolateral (AL) bundle, posteromedial (PM) bundle, and meniscofemoral ligaments (MFLs):
PM bundle is tight in extension.
AL bundle is tight in flexion.
Anterior and posterior MFLs of Humphrey and Wrisberg come from the posterior horn of the lateral meniscus and attach to the PCL anteriorly and posteriorly, respectively.
PCL attaches to the medial femoral condyle at the anterior cartilage margin and inserts 1.0–1.5 cm below the joint line on the posterior tibia in close proximity to the popliteal artery.
Etiology
ACL
Acute injury usually occurs as a noncontact pivoting injury.
Accompanied by feeling a “pop” with pain and swelling.
PCL
More commonly injured in trauma than sports-related injuries; concomitant injuries are common, particularly to the posterior lateral corner (PLC).
Patients rarely complain of a “pop” or instability but rather posterior knee pain with decreased knee flexion.
Pathophysiology
ACL
Acute ACL tears are often associated with lateral meniscal tears and medial collateral ligament injury (MCL).
Chronic ACL-deficient knees also often have meniscal tears secondary to knee instability and chondral injuries.
More common in women compared with men (4.5:1):
Due to valgus leg alignment, landing biomechanics (landing more in extension, higher valgus movement), more quadriceps dominant, smaller ligaments, smaller notches, hormone levels, and genetic factors related to collagen production
PCL
Patients more likely to be symptomatic if they have a combined ligamentous knee injury.
After the acute effects of the knee injury resolve, patients’ symptoms vary from little to severe impairment. Some only complain of instability while walking up inclines.
Radiography
ACL
Radiographs should be done initially and usually are normal.
Segond fracture may be seen which is pathognomonic for an ACL tear:
Due to an avulsion fracture of the proximal lateral tibia
MRI should be done to further evaluate for ACL tears, meniscal tears, subchondral injury, loose bodies, and bone bruising.
Bone bruising occurs from direct impact of the tibia and femur and classically occurs in the middle 1/3 of the lateral femoral condyle and posterior 1/3 of the lateral tibial plateau.
PCL
X-rays should include bilateral standing anteroposterior, flexion 45° weight bearing, lateral, and merchant views.
Evaluation of tibial subluxation is key as well as any fractures, particularly avulsion fractures.
MRI is very sensitive in acute PCL injury but less so with chronic tears:
Allows evaluation of the meniscus, articular surface, and other ligamentous structures.
Locations of bone bruises vary.
Classification
ACL
An effusion is usually seen and quadriceps avoidance gait is utilized where the patient does not actively extend the knee.
Lachman’s test is the most sensitive exam:
Grades I–III and A or B
Grade I is <5 mm translation.
Grade II is 5–10 mm translation.
Grade III is >10 mm translation.
A is when a firm end point is felt.
B with no endpoint.
Anterior drawer is another option and KT-1000 testing can be used to quantify anterior laxity.
Pivot shift is done by internally rotating the lower leg 20° and a valgus force applied while the knee is flexed:
Positive test when the tibial plateau reduces and a clunk can be felt at 20–30° of flexion.
PCL
Classification is based on chronicity (acute vs. chronic), associated knee injuries, and the amount of translation of the tibia.
Key to diagnosis is whether the injury is isolated or combined as treatment varies:
Isolated PCL injuries many times can be treated nonoperatively with good to excellent results.
Combined injuries many times have better prognosis with early surgical intervention. Commonly combined with other ligamentous injuries, fractures, vascular, and nerve injuries.
Knee dislocation should be suspected with ACL and PCL injury or any three-ligament disruption:
Vascular evaluation is important.
Chronicity is important as PLC scarring occurs if surgery is not undertaken within 3 weeks and more rotational instability may exist due to capsular stretching.
Posterior drawer test is the most accurate test:
At 90° of flexion a posterior directed fore is applied on the proximal tibia. Normally, an anterior step-off of 1 cm from the medial femoral condyle to the tibial plateau exists.
Classification is like with the ACL:
Grade I injury has a translation of 1–5 mm but maintains the anterior step-off.
Grade II translates 5–10 mm and the condyle and plateau are flush.
Grade III has >10 mm of translation, and the plateau is posterior to the condyle and is a complete tear.
Posterior sag test or Godfrey’s test is the evaluation of this step-off due to the weight of the tibia translating the plateau posteriorly.
Quadriceps activation test is when the knee is held in 60° of flexion and the patient contracts the quadriceps muscle to extend their knee:
In grade III tears, the tibia will reduce anteriorly.
The dial test or external rotation of the tibia is important to evaluate PLC injury as this can change the prognosis and intervention.
Evaluation of the meniscus as well as the other ligamentous structures is also very important as outlined previously.
Treatment
ACL
Nonoperative treatment—physical therapy and lifestyle modifications:
Appropriate in low demand and older individuals
ACL repair has high failure rates and poor outcomes; therefore, reconstruction of the ACL is done in younger, more active patients.
Proper anatomic tunnel placement is imperative to restore rotation stability.
The tunnels should be placed anatomically within the native footprints.
Single-bundle and double-bundle reconstruction are options:
Double bundle has shown to give improved rotational stability in laboratory evaluation and some clinical studies.
Knees without ACL reconstruction many times have worsening meniscal pathology and chondral injury, and early arthritic progression may be linked:
Reconstruction is recommended in young, active patients.
Graft selection is controversial.
Options include bone patellar bone autograft, hamstring autograft, quadriceps tendon autograft, and allograft:
Bone patellar bone autograft allows bone healing and therefore may incorporate faster and is rigidly fixed:
Negatives include possible anterior knee pain from graft harvesting, patella fracture, or patella tendon rupture.
Hamstring autograft has a smaller incision and less anterior knee pain but fixation strength may be decreased:
Some concern for hamstring weakness in elite athletes and a “windshield wiper” effect because femoral fixation is further from the joint so the graft may rub in the tunnel and be impinged.
Quadriceps tendon autograft allows a large graft with possible bone fixation and good biomechanical properties.
Many different allografts can be used:
Take longer to incorporate and sterilization impairs its native properties and rarely disease transmission can occur.
PCL
Nonoperative versus operative treatment of isolated PCL injuries is controversial.
Rarer injury and therefore few larger long-term follow-up studies exist.
Isolated acute partial PCL injuries have good outcomes with nonoperative treatment.
A more aggressive treatment approach is suggested if the patient has persistent knee instability and pain due to the increased loading of the medial compartment in isolated complete tears.
PCL does have healing potential so some recommend extension bracing.
PCL avulsion fractures are less common and have more agreed upon treatment:
Minimally displaced fractures do well with brief immobilization.
Displaced fractures require operative intervention.
Multiple graft options exist as well as fixation techniques without any clear superior option.
Transtibial and tibial inlay procedures are arthroscopic versus open techniques, respectively, and each has their strengths and risks.
Single- versus double-bundle reconstruction is another area of controversy:
Most reconstructions currently involve the AL single bundle only.
The popliteal artery lies just posterior to the insertion of the PCL; great care must be taken during surgical intervention.
In chronic PCL-deficient knees, high tibial osteotomy can unload the medial compartment and increase the tibial slope to eliminate varus angulation and lessen the posterior tibial sag.
In isolated PCL and multi-ligamentous injuries, each patient must be dealt with individually as a wide array of injuries and outcomes exist.
Bibliography
1.
Amis AA, et al. Anatomy of the posterior cruciate ligament and the meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):257–63.
2.
Anderson AF, Snyder RB, Lipscomb AB Jr. Anterior cruciate ligament reconstruction. A prospective randomized study of three surgical methods. Am J Sports Med. 2001;29(3):272–9.
3.
Bathala EA, et al. Radiologic case study. Segond fracture. Orthopedics. 2007;30(9):689, 797–8.
4.
Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24(3):306–10.
5.
Chhabra A, et al. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(Suppl 4):2–10.
6.
Ciccotti MG, et al. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315–21.
7.
Freedman KB, et al. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2–11.
8.
Giffin JR, et al. Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med. 2004;32(2):376–82.
9.
Hensler D, et al. Correlation between femoral tunnel length and tunnel position in ACL reconstruction. J Bone Joint Surg Am. 2013;95(22):2029–34.
10.
Honkamp NJ, Ranawat A, Harner CD. Posterior cruciate ligament injuries in the adult. In: DeLee JC, Drez D, Miller MD, editors. DeLee & Drez’s orthopaedic sports medicine: principles and practice. Philadelphia: Elsevier; 2009. p. SECTION E.
11.
Honkamp NJ, Shen W, Okeke N, Ferretti M, Fu FH. Anterior cruciate ligament injuries in the adult. In: DeLee JC, Drez D, Miller MD, editors. DeLee & Drez’s orthopaedic sports medicine: principles and practice. Philadelphia: Elsevier; 2009. p. SECTION D.
12.
Hoshino Y, et al. Quantitative evaluation of the pivot shift by image analysis using the iPad. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):975–80.
13.
Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res. 2000;(372):50–63.
14.
Illingworth KD, et al. Relationship between bone bruise volume and the presence of meniscal tears in acute anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2014;22:2181–6.
15.
Kennedy JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior cruciate ligament. As determined by clinical and morphological studies. J Bone Joint Surg Am. 1974;56(2):223–35.
16.
Mair SD, et al. Incidence and location of bone bruises after acute posterior cruciate ligament injury. Am J Sports Med. 2004;32(7):1681–7.
17.
Meyers MH. Isolated avulsion of the tibial attachment of the posterior cruciate ligament of the knee. J Bone Joint Surg Am. 1975;57(5):669–72.
18.
Rabuck SJ, et al. Anatomic anterior cruciate ligament reconstruction with quadriceps tendon autograft. Clin Sports Med. 2013;32(1):155–64.
19.
Race A, Amis AA. The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech. 1994;27(1):13–24.
20.
Schulz MS, et al. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186–91.
21.
Sekiya JK, et al. Clinical outcomes after isolated arthroscopic single-bundle posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(9):1042–50.
22.
Seroyer ST, Musahl V, Harner CD. Management of the acute knee dislocation: the Pittsburgh experience. Injury. 2008;39(7):710–8.
23.
Shino K, et al. Conservative treatment of isolated injuries to the posterior cruciate ligament in athletes. J Bone Joint Surg Br. 1995;77(6):895–900.
24.
Thompson WO, Fu FH. The meniscus in the cruciate-deficient knee. Clin Sports Med. 1993;12(4):771–96.
25.
Viskontas DG, et al. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med. 2008;36(5):927–33.
26.
West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207.
27.
Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93(16):1510–8.
2 Collateral Ligament Injury
Justin Arner6
(6)
Labrum is University of Pittsburgh Medical School, Arner is Orthopedic Residency UPMC, University of Pittsburgh Medical Center, 3200 South Water Street, Pittsburgh, PA 15203, USA
Take-Home Message
Medial Knee
Superficial MCL is the primary static stabilizer against valgus and external rotation stress.
Isolated MCL injuries have valgus laxity at 30°.
Combined MCL and ACL injuries have valgus laxity at 0°.
Lateral Knee
Injuries occur by an excessive valgus force, external tibial rotation, or hyperextension.
The lateral collateral ligament (LCL) is the main stabilizer, is rarely an isolated injury, but commonly is seen with posterolateral corner (PLC) injury:
Varus stress and dial testing helps distinguish if a combined injury exists.
Definitions
Main structures include MCL and LCL.
Anatomy
Medial Knee
Major static stabilizers: superficial MCL, deep MCL, and posterior oblique ligament.
Dynamic stabilizers: pes anserinus, semimembranosus, medial head of the gastrocnemius, and vastus medialis.
These structures can be divided into three layers:
Layer I: sartorial fascia
Layer II: superficial MCL
Superficial MCL originates at the medial femoral epicondyle and broadly inserts 5–7 cm below the joint line on the anteromedial tibial metaphysis:
Primary restraint to valgus stress
Layer III: capsule and deep MCL
Deep MCL is a thickening of capsule and has the same origin but inserts into the meniscus (meniscofemoral) and then runs from the meniscus to the tibia (meniscotibial).
Layers II and III conjoin posteriorly to form the posteromedial corner.
The posterior oblique ligament runs from the adductor tubercle of the femur to the tibia and posterior capsule.
The MCL is supplied by the superior medial and inferior medial geniculate arteries.
Lateral Knee
Separated in three layers:
Layer I—iliotibial (IT) band and the biceps femoris tendon:
The common peroneal nerve lies between layers I and II.
Layer II—patellar retinaculum and patellofemoral ligament.
Layer III includes a superficial region made up of the LCL, fabellofibular ligament, and the deep region which includes the arcuate ligament, coronary ligament, popliteus tendon, popliteofibular ligament, and the capsule:
Between the two subsets in layer 3 lies the inferior lateral geniculate artery.
PLC is made up of the LCL, popliteus tendon, popliteofibular ligament, lateral capsule, arcuate ligament, and the fabellofibular ligament.
The LCL originates on the lateral femoral condyle, proximal and posterior to the popliteus insertion, and inserts on the anterolateral fibular head:
Its attachment to the fibula is the most anterior structure as the popliteofibular ligament and biceps femoris attachments lay posterior to the LCL, respectively.
The primary function of the LCL is resisting varus stress with a secondary role of resisting posterolateral rotation when the knee is in less than 50° of flexion.
Because its axis is behind that of the knee axis, it is tight in extension and lax in flexion.
The blood supply is via the superolateral and inferolateral geniculate arteries.
Etiology
Medial Knee
The MCL is a valgus stabilizer.
Commonly injured when the knee is forced into valgus and external rotation.
Commonly seen in combined injuries with the anterior cruciate ligament (ACL) and meniscus.
Usually a direct blow to the lateral knee leads to a more severe injury compared with a noncontact injury.
Rupture more commonly occurs at the femoral insertion which is also the location where the MCL heals best.
Chronic MCL deficiency can lead to calcification of the MCL femoral insertion site and is known as Pellegrini-Stieda syndrome.
Lateral Knee
Lateral knee injuries most commonly is seen in motor vehicle accidents and athletic injuries and occurs by an excessive varus force, external tibial rotation, or hyperextension.
The lateral collateral ligament (LCL) is the main stabilizer of the lateral knee and is rarely an isolated injury but commonly is seen with posterolateral corner (PLC) injury.
Pathophysiology
Medial Knee
The typical history of MCL injury is hearing a “pop” and medial joint line pain with difficulty ambulating.
On exam, patients are classically tender on the medial knee with an effusion and swelling.
Valgus stress testing at 30° of knee flexion isolates the superficial MCL.
If medial laxity at 0° of knee flexion as well, a posterior medial capsule or cruciate ligament injury is considered.
It is important to evaluate the patient for other ligamentous, meniscal, and chondral lesions.
A neurovascular exam should be performed with special attention to the saphenous nerve distribution.
Lateral Knee
Symptoms of injury to the LCL is lateral joint line pain, swelling, difficulty ascending and descending stairs, instability with knee extension, and problems with cutting or pivoting.
On exam, patients classically walk in hyperextension or a varus thrust gait and have lateral joint line tenderness.
Isolated LCL injury:
Instability to varus stress test at 30°
LCL combined with ACL and/or PCL injury:
Varus instability at both 0 and 30°.
If increased tibial external rotation is seen at 30°, a combined PLC and LCL injury is likely.
Dial test—patient prone and applying external rotation stress on tibia:
>10° tibial external rotation asymmetry at 30°: isolated PLC injury
>10° external rotation asymmetry at 30° and 90°: PLC and PCL injury
A complete neurovascular exam is critical as common peroneal nerve injuries do occur.
Radiographs
MCL: AP and lateral X-rays and MRI to identify the location and severity of MCL injury as well as other knee structures.
LCL: similar radiographic analysis is recommended as well as a varus stress view.
Classification
Medial Knee
Three grades based on the amount of valgus opening:
Grade I: 1–5 mm
Grade II: 5–10 mm
Grade III: gross laxity with no endpoint and >10 mm of gapping
Lateral Knee
Injuries of the LCL and PLC are graded by the amount of lateral opening on varus stress:
Grade I is 0–5 mm.
Grade II is 5–10 mm.
Grade 3 is >10 mm.
Grade I and II are partial tears while grade III is a complete tear.
Treatment
Medial Knee
Treatment is based on the grade of injury.
Grade I injuries:
Rest, nonsteroidal anti-inflammatories (NSAIDs), and immediate physical therapy.
Return to play is usually 5–7 days.
Isolated grade II and III injuries:
Rest, NSAIDS, therapy, and hinged knee bracing.
Return to play is typically 2–4 weeks for grade II injuries and 4–8 weeks for grade III injuries.
Functional knee bracing for prophylactic MCL treatment has been shown to be successful in football.
Operative intervention is utilized with combined ligamentous injuries and grade III injuries with continued instability after nonoperative treatment.
Repairing the MCL is successful in acute avulsion injuries where suture anchors are used.
MCL reconstruction is utilized in chronic instability or if repairing the MCL is not possible.
Graft types include semitendinosus, hamstring autograft, tibialis anterior, or Achilles allograft.
Lateral Knee
Nonoperative treatment is many times successful in isolated grade I or II LCL injury and includes immobilization and functional rehabilitation.
Return to sports usually possible in 6–8 weeks.
Operative Indications
Grade III LCL injuries.
Rotatory instability (combined LCL and PLC).
Posterolateral instability (combined LCL/PCL and ACL/PCL injuries).
The proper order of repair/reconstruction of these combined knee injuries is not well defined and is a source of controversy.
Outcomes are improved when operative intervention occurs in the acute setting.
Avulsed LCL injuries have shown good outcomes with suture anchor fixation and direct suture repair of midsubstance injuries within 2 weeks of injury.
When reconstruction is required in isolated LCL injury, patellar tendon autograft has shown good outcomes.
In combined LCL and popliteofibular ligament reconstruction, the Larson technique can be used where the hamstring graft is passed through a fibular head bone tunnel and is fixed to the lateral femur to create a figure of eight.
Another option for this same injury is a transtibial double-bundle technique:
Achilles tendon allograft is fixed to the femoral epicondyle and half of the tendon is fixed to the fibular head with sutures through a bone tunnel and the other half is fixed to the posterior tibia.
Careful physical exam is critical for quick diagnosis of combined injuries which improves outcomes.
Bibliography
1.
Albright JP, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2–11.
2.
Chen FS, Rokito AS, Pitman MI. Acute and chronic posterolateral rotatory instability of the knee. J Am Acad Orthop Surg. 2000;8(2):97–110.
3.
Cooper JM, McAndrews PT, LaPrade RF. Posterolateral corner injuries of the knee: anatomy, diagnosis, and treatment. Sports Med Arthrosc. 2006;14(4):213–20.
4.
Halinen J, et al. Operative and nonoperative treatments of medial collateral ligament rupture with early anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med. 2006;34(7):1134–40.
5.
Hughston JC, et al. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–9.
6.
Kannus P. Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;(226):103–12.
7.
Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17(1):83–8.
8.
LaPrade RF, et al. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854–60.
9.
LaPrade RF, et al. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000–10.
10.
Singhal M, PJ, Johnson D. Medial ligament injuries. In: DeLee JC, Drez D, Miller MD, editor. DeLee & Drez’s orthopaedic sports medicine: principles and practice. Philadelphia: Elsevier; 2009. p. section C.
3 Chronic Exertional Compartment Syndrome
Scott Kling7
(7)
Sports Medicine, University of Pittsburgh Medical Center, 3200 South Water Street, Pittsburgh, PA 15203, USA
Take-Home Message
The anterior compartment is most commonly affected, followed by the lateral compartment. Posterior compartment involvement is rare and has less predictable surgical outcomes.
Diagnostic criteria for compartment pressure measurement are the following: (1) resting pressure >15 mmHg, (2) immediate postexercise pressure >30 mmHg, and (3) continuous postexercise pressure that does not return to resting level or fall below 15 mmHg after 15 min after completing activity.
The mainstay of treatment is surgical decompression fasciotomy of the involved compartments. This can be performed using an open or endoscopic approach with similar results.
Definition
Exertional compartment syndrome is a relatively uncommon cause of leg pain in the athletic population.
It is defined as reversible muscle ischemia within one of the compartments of the leg that is induced by exercise and relieved by rest.
There are no permanent sequelae in the affected tissues.
Epidemiology
Exertional compartment syndrome is found most commonly in male in the third decade of life.
Athletes who perform significant amounts of running are frequently affected.
Anatomy
There are four compartments of the leg (anterior, lateral, superficial posterior, and deep posterior):
The anterior compartment contains the anterior tibial artery and the deep peroneal nerve.
The lateral compartment contains the superficial peroneal nerve.
The superficial posterior compartment contains the sural nerve.
The deep posterior compartment contains both the posterior tibial and peroneal arteries and the posterior tibial nerve.
The anterior compartment is most commonly affected in approximately 70 % of cases.
The lateral compartment is involved in 10 % of cases.
Posterior compartment involvement is rare and has less predictable surgical outcomes.
Pathophysiology
The exact mechanism by which exertional compartment syndrome induces pain remains unknown.
It is theorized that increasing blood perfusion, muscle hypertrophy, and increased interstitial fluid volume within a confined fascial compartment lead to significantly elevated pressures. Accordingly, there is decreased venous return, leading to impaired tissue oxygenation and ischemia. Individual muscle cells are unable to clear the metabolic waste products at a sufficient rate, and they accumulate within the cell membrane.
Additionally, fascial hernias are suspected to be causal in some cases, with an incidence of 40 % during surgical decompression. The most common location is the intermuscular septum between the anterior and lateral compartments at the exit of the superficial peroneal nerve.
Presentation
Patients generally present complaining of aching or burning leg pain that is induced by running. It is both predictable and reproducible in nature, often beginning 10 min or so after the onset of exercise, escalating during activity, and slowly resolving after a 30 min period of rest.
The patient may note a recent increase in training intensity that exceeds their threshold for generating symptoms.
There is no history of trauma.
Bilateral involvement is common.
Pain is localized to the involved compartment.
Occasionally, patients will complain of distal neurologic findings in addition to pain, specifically numbness, paresthesias, or weakness in the affected nerve distribution. However, the physical exam is frequently normal.
The diagnosis is commonly confused with medial tibial stress syndrome, colloquially referred to as “shin splints.” However, in medial tibial stress syndrome, pain is worst at the onset of exercise and tends to decrease during training.
Imaging
Plain film radiographs, magnetic resonance imaging, and bone scan are often normal in patients with exertional compartment syndrome. However, these studies are often helpful in excluding other diagnoses such as tibial stress fracture and medial tibial stress syndrome.
Evaluation
Exertional compartment syndrome is definitively diagnosed by measuring compartment pressures. Measuring compartment pressures during exercise proves difficult and impractical.
Per Pedowitz criteria, three pressures must be measured for each suspected compartment: resting pressure, immediate postexercise pressure, and continuous postexercise pressure for 30 min. Diagnostic criteria are the following: (1) resting pressure >15 mmHg, (2) immediate postexercise pressure >30 mmHg, and (3) continuous postexercise pressure that does not return to resting level or fall below 15 mmHg after 15 min after completing activity.
While the anterior and lateral compartments may be tested fairly reliably, the deep posterior compartment can be challenging.
Alternative methods of diagnosis are being explored. Near-infrared spectroscopy will show increased deoxygenation of muscle during activity with delayed normalization after activity cessation. T2-weighted magnetic resonance imaging during exercise will demonstrate increase signal in the involved compartments when compared to normal surrounding tissue. SPECT bone scan has also demonstrated potential to localize an ischemic compartment.
Treatment
Nonoperative treatment includes activity modification and antiinflammatory medications but often proves ineffective. Patients generally do not improve unless they completely eliminate the inciting activity.
The mainstay of treatment is surgical decompression fasciotomy of the involved compartments, most commonly anterior and lateral. This can be performed using an open or endoscopic approach with similar results.
The open approach involves a longitudinal incision over the anterolateral leg, identification of the intermuscular septum, and proximal and distal fascial division of both compartments. Careful attention should be paid to localize and protect the superficial peroneal nerve.
Posterior compartments may be accessed through an extensile anterolateral dissection or a separate medial approach. The superficial compartment is easily decompressed, and the deep compartment must be reached by undermining the soleus to access the posterior tibial margin.
Many surgeons advocate an individual release of the tibialis posterior muscle as well.
Most patients are able to return to sports postoperatively within 4 weeks.
Success rates are greater than 80 % when involving anterior and lateral compartments but fall to between 50 and 65 % when involving posterior compartments, particularly the deep.
Patients note a high level of pain relief and satisfaction with the surgical result.
Complications
The most common complication is recurrence of symptoms, which has been found in 7–17 % of operative cases. This is often attributed to incomplete fasciotomy and not specifically releasing the tibialis posterior during deep posterior decompression.
Less frequent complications include wound infection, nerve injury (most commonly the superficial peroneal), and deep venous thrombosis.
Bibliography
1.
Edwards P, Myerson MS. Exertional compartment syndrome of the leg: steps for expedient return to activity. Phys Sportsmed. 1996;24:31–46.
2.
George CA, Hutchinson MR. Chronic exertional compartment syndrome. Clin Sports Med. 2012;31(2):307–19.
3.




Hislop M, Batt ME. Chronic exertional compartment syndrome. Br J Sports Med. 2011;45:954–5.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






