Fossil of Pikaia gracilens, a 505-million-year-old creature, found in the Burgess Shale fossil beds in Canada. There is evidence of a notochord, a dorsal nerve, and myotome-like structure. Pikaia was originally thought to be a chordate which would thus make it an early vertebrate ancestor (Courtesy of Smithsonian Institution)
1.1.2 Development of the Vertebrae and Intervertebral Disc
The vertebrae develop from individual ossification centers which are well documented historically (Kerkring 1717; Albinus 1737; Rambaud and Renault 1864). Probably the most detailed report in the twentieth century was by Peacock (1951). The reader is urged to review the latter report for more details; the developmental biology of the intervertebral disc and the vertebrae is discussed in great detail in Chap. 3.
The vertebral bodies are formed by fusion of sclerotome from two adjacent somites: thus, tissue from the caudal portion of one sclerotome fuses with cranial sclerotome of the succeeding somite. The dense connective tissue of the two halves of each sclerotome becomes two centers of chondrogenesis. At each putative vertebrae, two more chondrogenic centers appear laterally and grow backwards to form the cartilage precursor to the neural arch. During this phase of development, the notochord becomes compressed by the pressure exerted by the cartilage and may persist for a while as a “mucoid streak.” However, between the developing vertebrae, notochordal tissue is retained and subsequently forms the intervertebral disc. At these sites, notochordal cells become enclosed in a dense ring of connective tissue, the putative annulus fibrosus. Noteworthy, some notochordal cells may remain in the cartilage; at a later time, cells buried in the bone of the centrum may serve as a site for tumor formation (see Chaps. 3 and 17). The nucleus pulposus is thus formed early in fetal life from notochordal elements and grows rapidly in late fetal life and early infancy. By birth, it occupies half of the intervertebral space in the lumbar region, and by 1 year it occupies almost three quarters of the space. It is thought that there is some remodeling of the intervertebral space early in life (Taylor 1975).
By the seventh week of life, the cartilage undergoes endochondral ossification. Dorsal and ventral blood vessels invade the two cartilage anlagen and trigger their replacement with bone. Subsequently, the anterior and posterior portions of the calcified centrum fuse. Along the anterior and lateral periphery of the vertebrae, cartilage plates appear to form the apophysis. This is the site for insertion of the fibers of the annulus fibrosus. As the centrum ossifies, the cartilage anlage of the neural arch is replaced by bone. The two sides of the arch fuse and then join together with the centrum. The process begins in the upper cervical region and extends caudally. The laminae are also formed in cartilage—they join together after birth and then fuse with the rest of the vertebrae between the third and seventh years of life. Vertebrae growth is mediated by chondrogenic activity at the growth plate. Actually, as the centrum has two centers of growth, it should be labeled as a synchondrosis. Histologically, prior to closure in the 17th–25th year, a well-defined zone of hypertrophic chondrocytes is visible. Once growth has ceased, the only remaining cartilage is the endplate.
1.2 Anatomical and Molecular Structure of the Intervertebral Discs
Medieval anatomists were the first to recognize that the vertebrae were separated by soft “gristle”-like structures, the intervertebral discs. In his intricate drawings of the spine, Winslow (1776) provided a detailed description of the disc, which considering the limitations posed by the distortions of hand lenses was remarkably accurate. Another analysis of spinal anatomy and the intervertebral disc in health and disease was performed by one of the most prolific anatomists of the nineteenth century, Hubert von Luschka. In his monograph Die Halbgelenke des menschlichen Körpers (1868), von Luschka described the gross and microscopic structure of the intervertebral discs from birth to death. Almost at the same time, Humphrey (1858) in his book A Treatise on the Human Skeleton provided a detailed description of each of the discs. He reported the looping fibrils of the annulus fibrosus and noted the absence of blood vessels in the nucleus and inner annulus fibrosus. Studies of age changes in the disc were subsequently noted by Henle (1872), Poirier and Charpy (1899), Fick (1904) and Petersen (1930), and Bohmig (1930). As far as we can tell, the earliest comment on the relationship of the disc to the notochord was reported by the Austrian anatomist Schaffer early in the twentieth century (1910).
1.2.1 Form and Function of the Intervertebral Discs
Depending on age, time of day, occupation, and disease state, the discs make up approximately 15–20 % of the length of the spinal column. Aside from absorbing biomechanical forces, each disc permits movement of the spinal column. Undoubtedly, flexibility decreases with age, while spinal movements at all stages of life can be severely limited by disease. Since vertebrae themselves are relatively inelastic, movement in the spine is mediated notably by the tissues of the intervertebral disc. Although the mobility of contiguous vertebrae (motion segments) can be viewed as limited, the integrated motion of the 33 intervertebral discs together with movement at the zygapophyseal joints permits all of the critical movements of the spine without compromising nerve or muscle function.
The famous English anatomist Henry Gray (1827–1861) classified articulations between vertebrae as “amphiarthroses in which the contiguous bony surfaces are either connected by broad flattened discs of fibrocartilage, of a more or less complex structure.” By definition, these joints permit very little motion. Shapiro et al. (2012) compared the structure-function relationships of both the intervertebral disc and synovial joints. On first consideration, the intervertebral disc could be seen as being very different from the generic synovial joint. However, on reflection, the separate tissues of the intervertebral disc are very similar to that of the diarthrodial joint: both types of joints are lined by cartilage, they are limited by an external ligament, and the joint space contains molecules that promote lubrication (lubricin and hyaluronan) and elevate the osmotic pressure (aggrecan). Indeed, even the presence of a band of nucleus pulposus tissue across the joint is not out of line with what is known of complex diarthrodial joints that contain cartilage and fibrocartilage discs and menisci. Related to the function of the nucleus pulposus and the inner annulus, it is not yet clear whether a distinct synovial-like membrane exists in the intervertebral disc. Whether inner annulus is derived from the notochordal sheath has not been determined. Nevertheless, like the cells of the synovium, the resident disc cells do have the ability to mount a robust defense against bacterial attack (Nerlich et al. 2002; Jones et al. 2008).
In terms of movement, the current classification of the disc as an amphiarthrosis would indicate very limited mobility. However, biomechanical studies of the motion segment with or without contributions from the zygapophyseal joints indicate that there is wide range of motion between vertebrae. Moreover, the actual movement of the cervical, thoracic, and lumbar vertebrae includes flexion-extension, axial rotation, and lateral bending, as well as translatory motions. These three-dimensional movements are more in line with those of a diarthrodial joint rather than an amphiarthrosis where movements are slow and motion is limited. Probably the major difference between appendicular diarthrodial joints and the axial intervertebral joints lies in their development. Although the joints originate from different mesenchymal elements, the nucleus pulposus is derived from a unique embryonic tissue, the notochord; deletion studies indicate here too there are considerable similarities in the expression of genes that govern organ development and maturation. Recent investigations indicate that joint formation and even function are dependent on the expression of a number of genes including those of the Hox family, BMPs, and GDF5 (Brunet et al. 1998; Archer et al. 2003; Pacifici et al. 2005). Indeed, deletion of Ext1 influences not just the development of limb joints but also the formation of the intervertebral disc (Mundy et al. 2011). This topic is considered further in Chap. 3.
Based on the overt structural and functional similarities between the intervertebral disc and the synovial joint and recognizing that while some differences exist between these articulations, it would seem logical to place the disc in the same grouping as the diarthrodial joint. Further, since the intervertebral motion segment displays movement in three dimensions and the spine itself provides further rotatory movements, Shapiro et al. (2012) were of the opinion that it should be classified not as an amphiarthrosis, “a slightly moveable joint,” but as a complex polyaxial joint.
1.2.2 Spinal Curvature
While the intervertebral discs and the zygapophyseal joints provide sites for vertebral motion, the overall shape of the spine as well as curvatures in specific regions of the spine is dependent on intrinsic genetic factors as well as biomechanical forces mediated through the pull of muscles, ligaments, and gravity. Encoded curvatures are seen in the cervical, lumbar, and sacral regions of the spine. On adoption of a vertical stance, and with maturation, these curvatures become more distinct (Fig. 1.1c). However, about 2–3 % of the population exhibit deficits in axial curvature, which vary from simple bending with little functional implications to excessive bending which impacts not just locomotor activities, but other critical functions associated with the spinal nerves. The “hunchback” spine, kyphosis, is due to excessive posterior bending of the thoracic motion segments (Fig. 1.2a); when the cervical and lumbar anterior spinal curvatures become excessive, this condition is termed lordosis (Fig. 1.2b). While these latter conditions are deviation in the anterior-posterior (cephalic-caudal) axis of the spine, abnormal bending is also seen in the lateral (side-to-side) dimensions. Scoliosis can affect any part of the spine; the most common regions are in the thoracic and the lower lumbar spine. These exaggerated musculoskeletal warps in spinal architecture are evident almost entirely in human populations even in royalty (Richard III); their occurrence in rodents is infrequent. Thus, from an experimental viewpoint, rodents and lagomorphs make useful models to investigate the molecular control of spinal curvature.
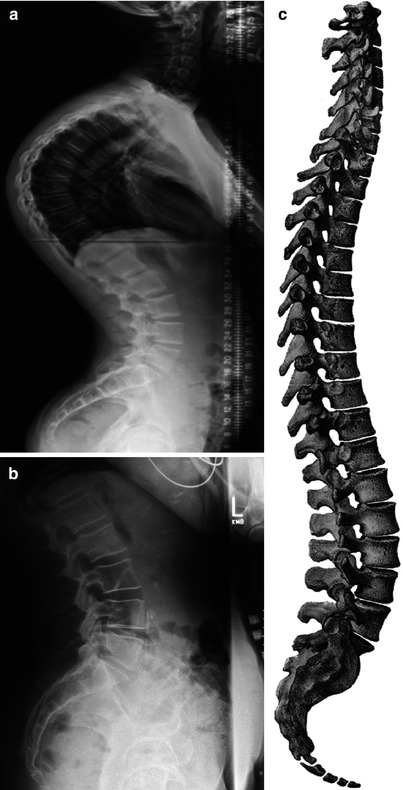
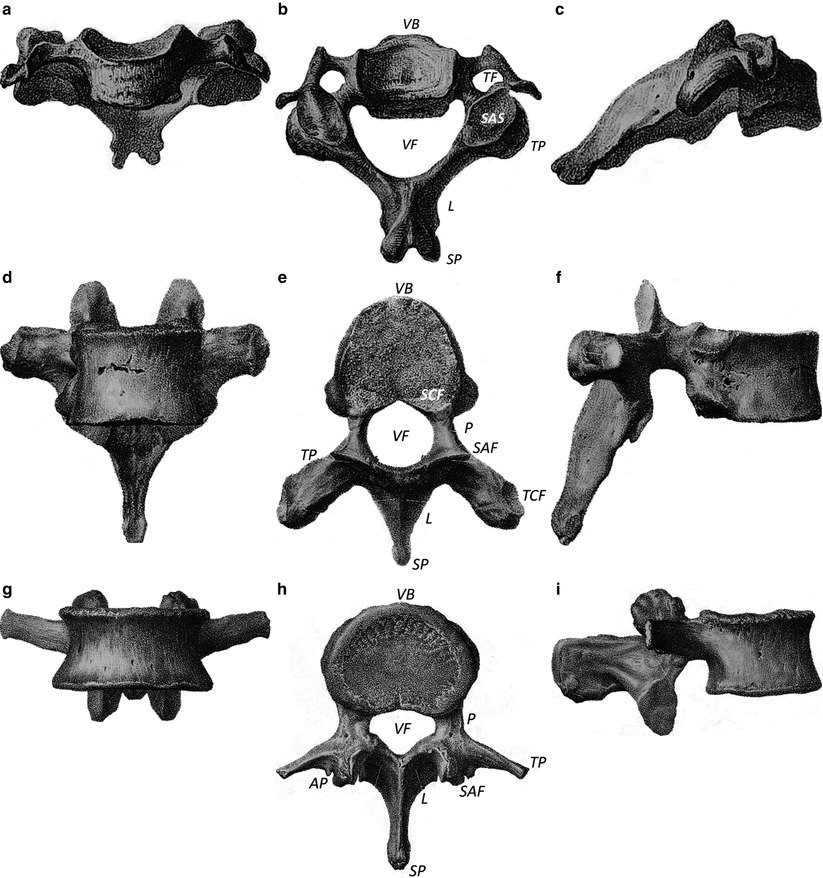

Fig. 1.1
Spinal curvature: kyphosis and lordosis. Anterior-posterior radiographs of the spine showing (a) kyphosis (excessive posterior bending of the thoracic motion segments) and (b) lordosis (extreme anterior bending of the lumbar and often the cervical spine). (c) The complete spine showing the natural curvature in the cervical, lumbar, and sacral regions (From Bougery and Jacob (1833). Plate 6)

Fig. 1.2
Human vertebrae. (a–c) show vertebrae from the cervical spine (below C2); (d–f) show vertebrae from the thoracic region of the spine; (g–i) show lumbar vertebrae. a, d, and g anterior-posterior aspects of the vertebrae; b, e, and h are superior views; c, f, and i are lateral views of the spine. VB vertebral body or centrum, P pedicle, L lamina, TP transverse process, VF vertebral foramen, SP spinous process, TF transverse foramen, SAS superior articular surface, SCF superior costal facet, TCF transverse costal facet, AP accessory process, SAF superior articular facet (From Bougery and Jacob (1833). Plates 8 and 9)
Clinical analysis of the types of abnormal spinal bending indicates that the most common form of this condition is idiopathic, i.e., of unknown origin. Nevertheless, the etiology of this condition is likely to be multifactorial as both environmental and genetic factors have been implicated. A second form of scoliosis is neuromuscular which is secondary to other conditions, such as cerebral palsy or a myopathy. In the elderly, abnormalities in axial bending are often due to degenerative disc disease and spondylolisthesis. Possibly, the most intriguing form of scoliosis is congenital in origin, a rare condition that is evident early in childhood (usually within the first 6–8 weeks). Radiographically, the spine exhibits fused vertebrae, single or multiple hemivertebrae, a vertebral bar, block vertebrae, and wedge-shaped or butterfly vertebrae. If left untreated, almost all of these congenital anomalies result in deformities and loss of normal function. Since the anomalies occur early in development, this form of scoliosis has been linked to patterning, particularly during the period of somitogenesis (Chal and Pourquie’ 2009).
As will be discussed in considerable detail in Chap. 3, somitogenesis occurs at a very early stage in development and is the process whereby the mesoderm of the developing embryo undergoes a carefully timed segmentation process; somites are generated that specify skeletal muscles, dermis, vertebrae, ribs, and annulus fibrosus. Very recent work by Pourquie’ (2011) has shown that the trigger for the rhythmic production of somites involves three major signaling pathways: Notch (Jiang et al. 2000), Wnt/β-catenin (Dequeant et al. 2006), and fibroblast growth factor (Benazeraf et al. 2010) which are integrated into a molecular circuit. The oscillatory activities of this circuit generate a highly coordinated developmental event that serves as a traveling wave of gene expression along the anterior-posterior axis of the developing embryo. Pourquie’ (2011) refers to this synchronized change in gene expression in the pre-mesodermal cells as the “segmentation clock.” Clearly any activity that interferes with coordinated gene expression and the development of the waves of gene oscillations will impact somitogenesis which in turn will influence the subsequent formation of the vertebrae and the intrinsic curvature of the axial skeleton. Although this system was developed from studies in mice, it is most likely that these new understandings will impact our understanding and ultimately the treatment of congenital scoliosis.
1.2.3 Gross Morphology and Dimensions of the Disc
The sizes of the discs in the human skeleton have been assessed by a number of investigators, especially in relationship with age, underlying conditions, and responses to surgery. Disc thickness can be assessed by radiography and other forms of imaging analysis. Frobin et al. (1997) made a determined effort to measure the disc and vertebrae height using archived radiographic measurements of the spine. This approach was complicated by a number of factors that included artifacts due to image distortion, axial rotation and lateral tilt, and even magnification. To account for these problems, algorithms were developed that generated dimensionless parameters. The study showed that lumbar vertebrae and discs were larger in males and females and in males there appeared to be no or little impact of age. More recently, magnetic resonance imaging (MRI) was used to provide direct information on the discs of seven healthy males aged 22–30 (Belavý et al. 2011).
Some general comments about disc dimensions are as follows. Disc height (cephalic-rostral dimensions) varies according to the spinal region. In the cervical spine, the disc height is about 3 mm, whereas in the lumbar spine, it is 9–17 mm; in the thoracic spine, the thickness is about 5 mm. In the cervical spine, the discs are thicker in the anterior region than posterior, thus helping to provide the curvature that is characteristic of the neck. In the thoracic spine, the discs are of constant thickness, whereas in the lumbar spine, they are again thickest anteriorly. Radiographs have been used to assess disc parameters in animals most commonly used in spine research (O’Connell et al. 2007).
1.2.4 Tissues of the Intervertebral Disc
The major functional role of the disc is mechanical: it allows movements between the axial and appendicular skeleton and the head; it accommodates applied loads; and to some extent the disc protects the spinal cord and nerve roots. The discs themselves are complex tissues comprising an outer circumferential ring of fibrocartilage, the annulus fibrosus which encloses a central proteoglycan-rich core, the nucleus pulposus. The nucleus is sandwiched caudally and cephalically by the cartilage endplates of the contiguous vertebrae. Since details of the biochemical, developmental, and biomechanical aspects of each of the disc tissues are provided in considerable detail in other chapters of this book, the sections below merely highlight the major characteristics of the endplate cartilage, nucleus pulposus, and the annulus fibrosus.
1.2.4.1 Annulus Fibrosus
As an introduction to these topics, it is worth noting that the annulus can be divided into an inner fibrocartilagenous region and an outer or peripheral fibrous zone (Souter and Taylor 1970). It was reported that the outer annulus fibrosus is composed of very well-defined collagen I fibers that bundle to form long parallel concentric lamellae. Marchand and Ahmad (1990) showed that the number of fiber bundles varies from 20 to 62. The thickness of lamellae varies both circumferentially and radially and increases markedly with age, location, and vertebral type. The central annulus fibers are inserted into the endplate cartilage, while those at the periphery are anchored to the vertebral bone. In terms of collagen organization and cell content, this region is not unlike tendon or ligament.
The inner annulus fibrosus represents roughly 50 % of the total radial thickness. Designated by some workers as the transitional zone, it differs substantially from the outer region. Compared with the outer annulus where the cells are elongated and fusiform and extend in the long axis of the fibrils, the cells of the inner annulus are spherical in shape and many resemble chondrocytes. These cells are few in number with short processes. A further difference between the inner and outer annulus is their chemical composition. The inner annulus contains collagens I and II. While aggrecan is present in both regions of the annulus, decorin and biglycan are found mainly in the outer annulus. The other protein of significance is elastin which accounts for 2 % of the dry tissue weight.
1.2.4.2 Nucleus Pulposus
The nucleus pulposus is derived from the notochord and notochordal cells remain in the tissue after birth and into adult life. During development, the nucleus is highly cellular: after birth, the number of cells is reduced; in the adult, the cell density is very low. The histology of the nucleus pulposus cells is unique and complex: large cells arranged mainly in clusters and separated by an abundant extracellular matrix. Among the large notochordal cells, much smaller cells possibly derived from the notochordal sheath can also be seen. The large cells appear to have numerous vacuoles, which has prompted some authorities to describe them as “physaliphorous.” Probably the most complete TEM analysis of the nucleus of the adult rabbits was described by Gan et al. (2003). These workers showed that the nucleus pulposus contained cell clusters embedded in a proteoglycan-collagen matrix. The cells exhibited a well-defined Golgi system, an extensive endoplasmic reticulum, and a complex vesicular system filled with beaded structures (proteoglycans). Neither necrotic nor apoptotic cells were evident. A remarkable finding was that the cells contain few if any mitochondria. A defining characteristic of the cells was the presence of numerous cytoplasmic processes.
With respect to the extracellular matrix, nucleus pulposus cells secrete aggrecan, as well as collagens I and II. The matrix also contains collagens IX and XI, and collagen X has also been reported to be present during degeneration. Because of the presence of aggrecan, the disc exhibits a high osmotic pressure; moreover, since it has no blood supply, the oxygen tension within the disc is very low. These limitations have prompted the Risbud group to note that nucleus pulposus cells “tune” their metabolism to the available oxygen supply (see Chap. 6 for details). In this case, nucleus pulposus cells evidence almost complete reliance on the glycolytic pathway to generate metabolic energy (Agrawal et al. 2007).
1.2.4.3 Endplate Cartilage
The caudal and cephalic ends of the disc are covered by a layer of cartilage, the endplate. This thin layer of hyaline cartilage is maximally thick in the newborn and thins with age; in the adult, the actual width is about 0.5–1 mm. It serves not just as an interface between the soft nucleus pulposus and the dense bone of the vertebrae, but as a biomechanical barrier that prevents the disc from applying pressure directly to the bone. It is the presence of the cartilage layer that provides the motion segment with its joint-like characteristics. Some authorities believe that the cartilage also plays a role in maintaining the viability of cells of the nucleus pulposus (Dahia et al. 2009). Structurally, the endplate resembles articular cartilage. Thus, it contains chondrocytes embedded in an aggrecan-rich and collagen II extracellular matrix. Although the cells do not undergo terminal differentiation, collagen X may be present in the central region of the endplate perhaps in relationship to focal areas of endochondral bone formation. The endplate transitions into bone through a region of calcified cartilage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








