Chapter 16 Infectious Diseases
Bronchitis
Patients often are primarily interested in alleviating symptoms caused by respiratory illness. Unfortunately, there is mixed evidence for the use of over-the-counter (OTC) and prescription cough medications. Dextromethorphan and codeine may be somewhat effective, although they have not been evaluated in randomized, double-blinded, placebo-controlled trials for acute bronchitis. Combination first-generation antihistamine-decongestant products may be effective for the cough associated with colds. Naproxen showed efficacy against cough in one upper respiratory model study (Sperber et al., 1992). Guaifenesin acts as an expectorant and may have some effect on cough by its mucus-thinning properties.
Chronic Bronchitis: Acute Exacerbation
KEY TREATMENT
Pneumonia
The most common microbiologic agent of pneumonia is often not isolated (Table 16-1). Furthermore, studies have shown that bacteriologic causes of pneumonia cannot be determined by radiographic appearance (i.e., “typical” vs. “atypical”). In the proper clinical setting, certain clinical microbes should be considered because they can affect treatment considerations and epidemiologic studies. These include Legionella spp., influenza A and B, and community-acquired methicillin-resistant Staphylococcus aureus (MRSA).
Table 16-1 Most Common Etiologies of Community-Acquired Pneumonia
| Patient Type | Etiology |
|---|---|
| Outpatient | |
| Inpatient (non-ICU) | |
| Inpatient (ICU) |
ICU, Intensive care unit.
∗ Influenza A and B, adenovirus, respiratory syncytial virus, and parainfluenza.
Modified from Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society–American Thoracic Society Consensus Guidelines on Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis 2007;44:S27-S72.
Certain diagnostic tests are performed based on clinical setting. Blood cultures are not routinely done in the outpatient setting but should always be done if the patient is being admitted to the hospital, ideally before antibiotics are given. The use of Gram stain and sputum culture remains controversial but can provide more evidence of a bacterial cause (e.g., many PMNs). If sputum cultures are being obtained, it is recommended that the physician have the patient expectorate directly into a specimen cup and have it sent immediately for processing. This can increase the yield of isolating Streptococcus pneumoniae among other respiratory pathogens. Other tests include urine antigen tests for S. pneumoniae, Legionella pneumophila serogroup 1, and nasal swab for influenza A and B. In young children, RSV, adenovirus, and parainfluenza in addition to influenza are common causes. Nasal swab for RSV and influenza can be rapidly done, but the other causes can be determined with viral cultures, serology, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR), although results usually are received after resolution of the acute symptoms.
Perhaps the most important decision for clinicians is to determine the location of treatment. The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) recommend use of the pneumonia severity index (PSI), which uses 20 variables to risk-stratify the patient into five mortality classes, or the CURB-65, which measures five clinical variables in this decision making. The CURB-65 may be the easiest and most convenient to use at the site of decision making. A score of 0 or 1 indicates treatment as an outpatient; a score of 2 requires hospital admission to the general medical ward; and a score of 3 or more indicates admission to an intensive care unit (ICU) (Box 16-1).
Treatment of CAP should be targeted toward the most likely etiology (Table 16-2). Outpatient therapy for patients who have no comorbidities and have not received antibiotics within the last 3 months includes doxycycline or a macrolide antibiotic. Use of a fluoroquinolone antibiotic (levofloxacin or moxifloxacin) should be reserved for patients with more complicated pneumonia and those requiring hospitalization. Patients who have comorbid conditions or recent antibiotic exposure, or who will be hospitalized, should receive a respiratory fluoroquinolone or combination therapy with a beta-lactam drug plus a macrolide, for 48 to 72 hours after fever abates (usually 5-7 days’ total therapy). If an organism is isolated, therapy may be narrowed to cover the causative agent. The clinician should consider longer therapy and appropriate antibiotics to cover for infection by less common organisms such as Staphylococcus aureus or Pseudomonas aeruginosa. If the patient has no more than one abnormal value (temperature <37.8° C, heart rate <100, respiratory rate <24, SBP >90, O2 saturation >90%, Po2 >60 on room air) and the patient is able to maintain oral intake and has a normal mental status, the clinician can safely switch to oral therapy and discharge the patient from the hospital. Unless the etiology of the pneumonia is known, the physician should switch to oral antibiotics in the same class as the intravenous antibiotics used.
Table 16-2 Guide to Empiric Choice of Antimicrobial Agent for Treating Patients with Community-Acquired Pneumonia (CAP)
| Patient Characteristics | Preferred Treatment Options |
|---|---|
| Outpatient | |
| Previously Healthy | |
| No recent antibiotic therapy | Oral-based β-lactam, macrolide,∗ or doxycycline |
| Recent antibiotic therapy† | A respiratory fluoroquinolone‡ alone, an advanced macrolide∗ plus high-dose amoxicillin,§ or an advanced macrolide plus high-dose amoxicillin-clavulanate.¶ |
| Comorbidities (COPD, diabetes, renal failure or congestive heart failure, or malignancy) | |
| No recent antibiotic therapy | An advanced macrolide∗ plus β-lactam or a respiratory fluoroquinolone |
| Recent antibiotic therapy | A respiratory fluoroquinolone‡ alone or an advanced macrolide plus a β-lactam∗∗ |
| Suspected aspiration with infection | Amoxicillin-clavulanate or clindamycin |
| Influenza with bacterial superinfection | Vancomycin, linezolid, or other coverage for MRSA or community-acquired MRSA |
| Inpatient | |
| Medical Ward | |
| No recent antibiotic therapy | A respiratory fluoroquinolone alone or an advanced macrolide plus a β-lactam†† |
| Recent antibiotic therapy | An advanced macrolide plus a β-lactam, or a respiratory fluoroquinolone alone (regimen selected will depend on nature of recent antibiotic therapy) |
| Intensive Care Unit (ICU) | |
| Pseudomonas infection is not an issue | A β-lactam†† plus either an advanced macrolide or a respiratory fluoroquinolone |
| Pseudomonas infection is not an issue but patient has a β-lactam allergy | A respiratory fluoroquinolone, with or without clindamycin |
| Pseudomonas infection is an issue‡‡ (cystic fibrosis, impaired host defenses) | Either (1) an antipseudomonal β-lactam§§ plus ciprofloxacin, or (2) an antipseudomonal agent plus an aminoglycoside## plus a respiratory fluoroquinolone or a macrolide |
| Nursing Home | |
| Receiving treatment in nursing home | A respiratory fluoroquinolone alone or vancomycin (for S. aureus including MRSA) plus a β-lactam (cefepime or piperacillin/tazobactam if Pseudomonas is suspected; ceftriaxone if Pseudomonas is not suspected) |
| Hospitalized | Same as for medical ward and ICU |
COPD, Chronic obstructive pulmonary disease; MRSA, methicillin-resistant Staphylococcus aureus.
∗ Azithromycin or clarithromycin.
† That is, the patient was given a course of antibiotic(s) for treatment of any infection within the past 3 months, excluding the current episode of infection. Such treatment is a risk factor for drug-resistant Streptococcus pneumoniae and possibly for infection with gram-negative bacilli. Depending on the class of antibiotics recently given, one or another of the suggested options may be selected. Recent use of a fluoroquinolone should dictate selection of a nonfluoroquinolone regimen, and vice versa.
‡ Moxifloxacin, levofloxacin, or gemifloxacin.
§ Dosage: 1 g orally (PO) three times daily (tid).
¶ Dosage: 2 g PO twice daily (bid).
∗∗ High-dose amoxicillin (1 g tid), high-dose amoxicillin-clavulanate (2 g bid), cefpodoxime, cefprozil, or cefuroxime.
†† Cefotaxime, ceftriaxone, ampicillin-sulbactam, or ertapenem.
‡‡ The antipseudomonal agents chosen reflect this concern. Risk factors for Pseudomonas infection include severe structural lung disease (e.g., bronchiectasis) and recent antibiotic therapy, health care–associated exposures or stay in hospital (especially in the ICU). For patients with CAP in the ICU, coverage for S. pneumoniae and Legionella species must always be considered. Piperacillin-tazobactam, imipenem, meropenem, and cefepime are excellent β-lactams and are adequate for most S. pneumoniae and H. influenzae infections. They may be preferred when there is concern for relatively unusual CAP pathogens, such as P. aeruginosa, Klebsiella spp., and other gram-negative bacteria.
§§ Piperacillin, piperacillin-tazobactam, imipenem, meropenem, or cefepime.
## Data suggest that older adults receiving aminoglycosides have worse outcomes.
¶¶ Dosage for hospitalized patients, 750 mg/day.
Data from Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27-S72.
KEY TREATMENT
Influenza
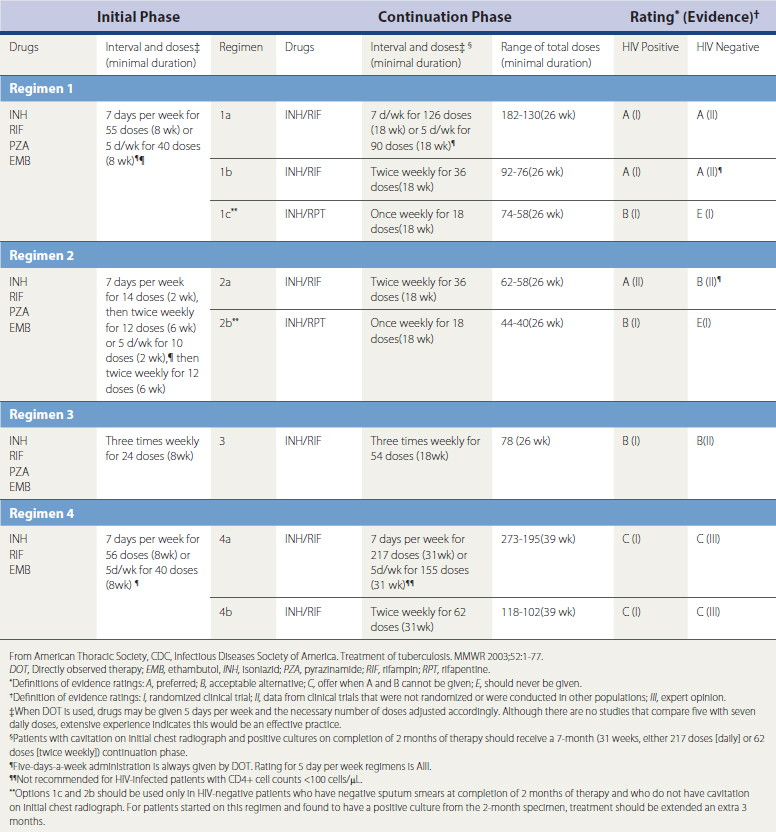
Influenza deserves special mention because it is an important cause of pneumonitis and can precede a bacterial pneumonia. Influenza viruses are medium-sized enveloped ribonucleic acid (RNA) viruses that consist of a lipid bilayer with matrix proteins with spiked surface projections of glycoproteins (hemagglutinins, neuraminidase) on the outer surface (Figure 16-1). Both influenza A and influenza B have eight segmented pieces of single-stranded RNA. The only difference between influenza A and B is that B does not have an M2 ion channel. Hemagglutinins, three types of which typically infect humans (H1, H2, H3), bind to respiratory epithelial cells and allow fusion with the host cell. Neuraminidase, consisting of two types (N1, N2), allows release of virus from the infected cells.
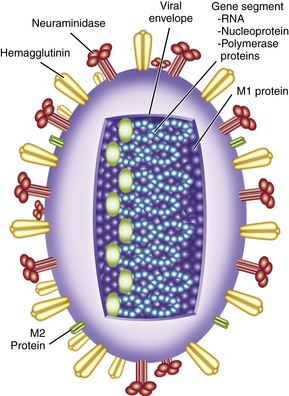
Figure 16-1 Schematic model of influenza A virus.
(From Treanor JJ: Influenza viruses, including avian influenza and swine influenza. In Mandell GL, Bennett JE, Dolin RD (eds). Mandell, Douglas, and Bennett’s Principles and Practices of Infectious Diseases, 7th ed. Philadelphia, Churchill Livingstone, 2010, p 2266.)
A unique aspect of influenza is that antigenic variation occurs annually. Antigenic shift is caused by a genetic reassortment between animal and human influenza strains, producing a novel virus that generally causes the worldwide pandemics. Influenza viruses circulate mostly among humans, birds, and swine. Sometimes; a human strain and an animal strain can intermingle and create a new, unique virus. This is what happened during spring 2009, heralding the most recent pandemic and creating “Novel H1N1 Influenza” (swine influenza). Genotype analysis of this strain determined that components came from an influenza virus circulating among swine herds in North America that combined with a virus circulating among ill swine in Eurasia, creating a new influenza strain capable of causing disease in humans. Because this virus had not previously infected humans, it had the potential to cause widespread morbidity and mortality worldwide. During pandemics, the U.S. Centers for Disease Control and Prevention (CDC) estimates an additional 10,000 to 40,000 deaths caused by influenza. Although higher than in nonpandemic years, mortality was significantly less than initially predicted in 2009.
The abrupt onset of fever, along with chills, headache, malaise, myalgias, arthralgias, and rigors during “flu season,” is sufficient to diagnose influenza. As the fever resolves, a dry cough and nasal discharge predominate. A rapid nasal swab or viral cultures can be used to confirm the diagnosis of influenza but is rarely needed. In fact, the sensitivity of these rapid tests can range from 50% to 70%, so a negative test does not rule out influenza. The primary care physician needs to determine if the patient has influenza or the common cold, because symptoms of both illnesses generally overlap (Table 16-3).
Table 16-3 Common Cold versus Influenza Symptoms
| Symptom | Common Cold | Influenza |
|---|---|---|
| Fever | Rare | Abrupt onset |
| Cough | Frequent, usually hacking | Frequent, usually severe |
| Sore throat | Frequent | Rare |
| Nasal congestion | Frequent | Rare |
| Sneezing | Frequent | Rare |
| Myalgia | Rare | Frequent |
| Headache | Rare | Frequent |
| Fatigue | Mild | Severe |
Treatment of influenza is generally not necessary because it is usually a self-limiting condition. Treatment should be reserved for those with comorbidities who present within 48 hours of symptom onset. Neuraminidase inhibitors (zanamivir and oseltamivir) prevent the release of virus from the respiratory epithelium and are approved for both influenza A and influenza B. The M2 inhibitors (amantadine and rimantadine) are approved by the U.S. Food and Drug Administration (FDA) for the treatment of influenza A because these drugs block the M2 ion protein channel, preventing fusion of the virus to host cell membrane (influenza B has no M2 ion channel). The use of M2 inhibitors is limited because of increasing resistance among influenza A viruses, as well as causing central nervous system (CNS) problems that are usually exacerbated in elderly persons, who are more likely to seek treatment for influenza (Table 16-4).
Table 16-4 Treatment and Chemoprophylaxis Recommendations for Influenza
| Agent/Group | Treatment | Chemoprophylaxis |
|---|---|---|
| Neuraminidase Inhibitors | ||
| Oseltamivir | ||
| Adults | 75-mg capsule twice daily (bid) for 5 days | 75-mg capsule once daily (qd) |
| Children (age >12 mo) | ||
| <15 kg | 60 mg/day divided into 2 doses | 30 mg qd |
| 15-23 kg | 90 mg/day in 2 doses | 45 mg qd |
| 24-40 kg | 120 mg/day in 2 doses | 60 mg qd |
| >40 kg | 160 mg/day in 2 doses | 75 mg qd |
| Zanamivir | ||
| Adults | Two 5-mg inhalations (10 mg bid) | Two 5-mg inhalations (10 mg qd) |
| Children | Two 5-mg inhalations (10 mg bid)(age >7 yr) | Twp 5-mg inhalations (10 mg qd)(age >5 yr) |
| M2 Inhibitors (Adamantadines)∗ | ||
| Rimantadine† | ||
| Adults | 200 mg/day as either a single daily dose or divided into 2 doses | 200 mg/day as either a single daily dose or divided into 2 doses |
| Children | ||
| 1-9 yr | 6.6 mg/kg/day (max, 150 mg/day) divided in 2 doses | 5 mg/kg qd, not to exceed 150 mg |
| >10 yr | 200 mg/day as either a single daily dose or divided into 2 doses | 200 mg/day as either a single daily dose or divided into 2 doses |
| Amantadine | ||
| Adults | 200 mg/day as either a single daily dose or divided into 2 doses | 200 mg/day as either a single daily dose or divided into 2 doses |
| Children | ||
| 1-9 yr | 5-8 mg/kg/day divided into 2 doses or as a single daily dose (max, 150 mg/day) | 5-8 mg/kg/day divided into 2 doses or as a single daily dose (max, 150 mg/day) |
| 9-12 yr | 200 mg/day divided into 2 doses | 200 mg/day divided into 2 doses |
∗ The amantadines should be used only when influenza A (H1N1) infection or exposure is suspected. The amantadines should not be used for infection or exposure to influenza A (H2N3) or influenza B.
† Rimantadine has not been approved by the U.S. Food and Drug Administration for treatment of children, although published data exist on safety and efficacy in the pediatric population.
Modified from Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children: diagnosis, treatment, chemoprophylaxis, and institutional outbreak management. Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1003-1032.
Prevention of influenza is generally with vaccination. Box 16-2 outlines patients at risk for influenza complications who should be vaccinated yearly. Although anyone wanting an influenza vaccine should be vaccinated, during periods of vaccine shortage, high-risk groups have priority. A well-matched vaccine can prevent influenza among 70% to 90% of adults and decrease work absenteeism. Conversely, a poorly matched vaccine only prevents influenza in 50% of healthy adults. Proper hand hygiene and covering one’s cough are two additional important components in preventing the spread of influenza virus.
Box 16-2 Groups at risk for Influenza Complications∗
Modified from Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children: diagnosis, treatment, chemoprophylaxis, and institutional outbreak management. Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1003-1032.
∗ Data suggest that the highest risk of both mortality and serious morbidity (e.g., hospitalization) occurs in severely immunocompromised patients (e.g., hematopoietic stem cell transplant patients) and very elderly (>85 years) residents of nursing homes; infants under age 24 months also have high hospitalization rates but lower case-fatality rates than the other two groups.
KEY TREATMENT
Systemic Viral Infections
Varicella and Herpes Zoster
Patients with primary varicella present with fever, headache, and sore throat. Generally within 1 to 2 days of onset of symptoms, a papulovesicular rash erupts diffusely. The classic description of the chickenpox lesion is “a dewdrop on a rose petal,” suggesting a central vesicle on an erythematous base. Lesions continue to appear for 5 to 7 days. All lesions going from papule to vesicle to crusted lesion takes about 2 weeks. Patients are considered to be infectious, primarily through respiratory secretions, during the 2 days before symptoms appear and until all lesions are crusted.
Epstein-Barr Virus and Cytomegalovirus
Splenic enlargement as part of this lymphoid hypertrophy can lead to splenic rupture (0.1% risk) (Dommerby et al., 1986). Athletes with infectious mononucleosis must be managed carefully to avoid their participation in sports that could result in abdominal trauma. Other risks associated with infectious mononucleosis include upper airway obstruction, asymptomatic transaminase elevation, thrombocytopenia, and rash after the administration of ampicillin or amoxicillin. Routinely obtaining transaminase levels in patients without clinical hepatitis is of little value and can increase the overall cost of management.
KEY TREATMENT
Tuberculosis
Presentation
Tuberculosis is most frequently manifested clinically as pulmonary disease, but it can involve any organ. Extrapulmonary TB accounts for about 20% of disease in HIV-seronegative persons but is more common in HIV-seropositive persons. Pulmonary TB typically manifest with fever, night sweats, chronic cough, sputum production, hemoptysis, anorexia, and weight loss. Chest radiographs in patients with pulmonary TB typically reveal upper-lobe cavitary lesions and can reveal infiltrates or nodular lesions, as well as lymphadenopathy (Figure 16-2). TB in the setting of advanced HIV co-infection does not generally manifest in the typical manner (Table 16-5).
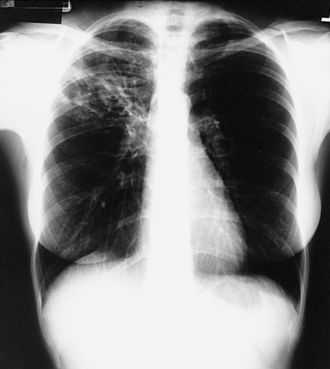
Figure 16-2 Chest radiograph showing right apical infiltrate typical of a patient with primary tuberculosis.
(From Fitzgerald D, Sterling T, Haas D. Mycobacterium tuberculosis. In Mandell GL, Bennett JE, Dolin R (eds). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed. Philadelphia, Churchill Livingstone, 2010, p 3141.)
Table 16-5 Clinical Manifestations of Active Tuberculosis in Early verus Late∗ Human Immunodeficiency Virus Infection
| Sign | Early | Late |
|---|---|---|
| Tuberculin test | Usually positive | Usually negative |
| Adenopathy | Unusual | Common |
| Pulmonary distribution | Upper lobe | Lower and middle lobes |
| Cavitation | Often present | Typically absent |
| Extrapulmonary disease | 10%-15% of cases | 50% of cases |
∗ For practical purposes, “early” and “late” may be defined as CD4+ cell counts >300 cells/mm3 and <200 cells/mm3, respectively.
Modified from Murray JF. Cursed duet: HIV infection and tuberculosis. Respiration 1990;57:210-220.
Treatment
Patients with AFB positive smears from sputum samples should be started on anti-TB therapy while awaiting results of PCR and cultures. The treatment of TB always uses multiple agents with anti-TB activity. Single agents should never be used. The standard first-line agents are isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) (Figure 16-3 and Table 16-6). If administered, INH should be given with pyridoxine (vitamin B6; 25-50 mg orally daily) to prevent neuropathy. Treatment of active pulmonary TB is generally for 6 months regardless of HIV status, but treatment may need to be extended in certain situations.
∗EMB may be discontinued when results of drug susceptibility testing indicate no drug resistance.
†PZA may be discontinued after it has been taken for 2 months (56 doses).
From Centers for Disease Control and Prevention (CDC). Treatment of tuberculosis. American Thoracic Society, CDC, and Infectious Diseases Society of America. MMWR 2003;52(RR-11):1-88.
Latent Tuberculosis Infection and Purified Protein Derivative
In the United States, latent tuberculosis infection (LTBI) is the most prevalent form of tuberculosis. LTBI is the term given to patients with a positive purified protein derivative (PPD) skin test without evidence of active TB. PPD has been used for more than 100 years and relies on delayed-type hypersensitivity (DTH) to M. tuberculosis cellular proteins. Because PPD relies on DTH, any factor that reduces the DTH affects the host response to PPD. The most common clinical example is use of corticosteroids, which blunt the DTH response and can complicate PPD interpretation. Therefore, PPD testing should not be performed while a patient is taking corticosteroids. Also, TB testing should be targeted to those with higher risk of infection and should not routinely be done in those with low risk (ATS/CDC, 2000).
The PPD can also give false-positive results in patients with previous bacille Calmette-Guérin (BCG) vaccination or with infection by other mycobacterial infections. In the United States, this may cause difficulties in testing immigrants from countries who routinely use BCG vaccination programs. However, previous BCG vaccination should not change the interpretation of the PPD or willingness to treat such individuals accordingly.
The DTH response can wane over time. To overcome this problem, nonreacting patients may undergo repeat PPD 1 week after their initial PPD. The diagnosis of LTBI is made by interpretation of a PPD and by ascertaining the patient’s risk factors for progression to active TB if left untreated (Box 16-3). Interpretation of the PPD should be based on the area of induration and not the area of surrounding erythema. Persons whose PPDs have converted from negative to positive within 2 years are presumed to have been infected recently. The decision to use PPD means treating the patient for LTBI if the PPD test is positive.