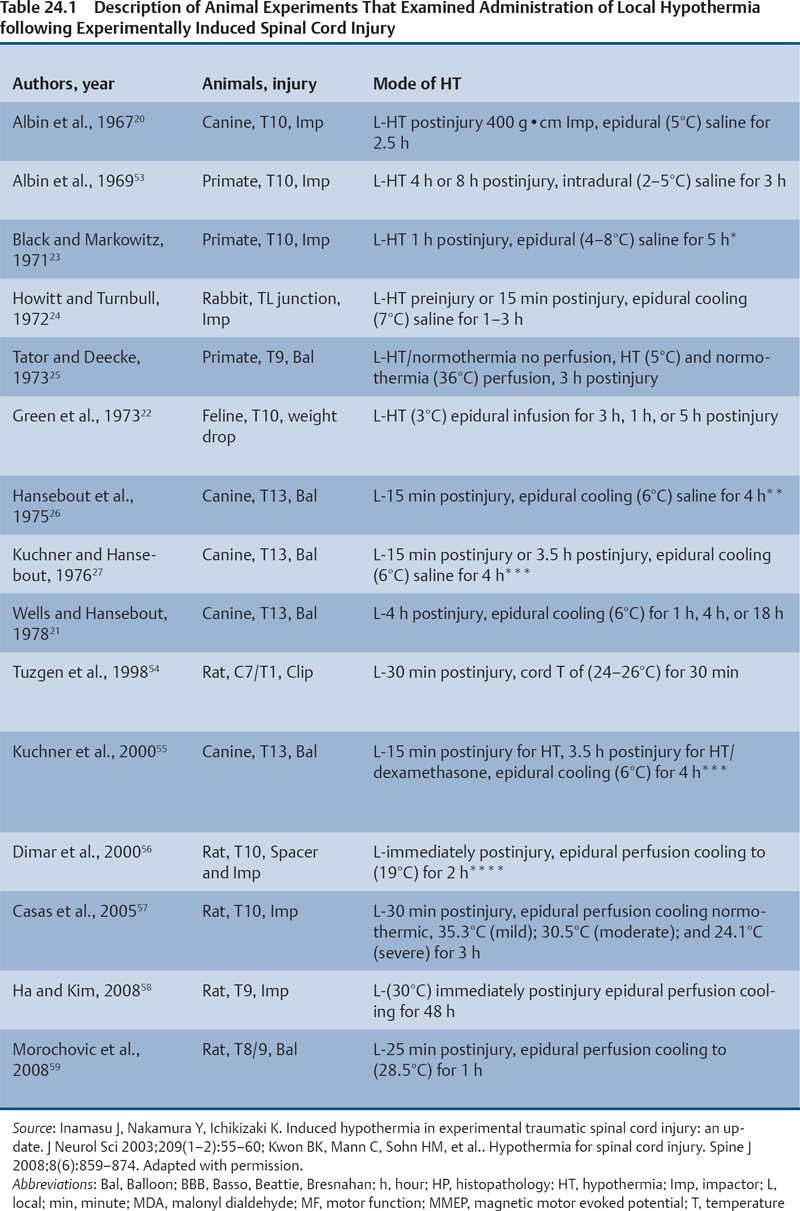
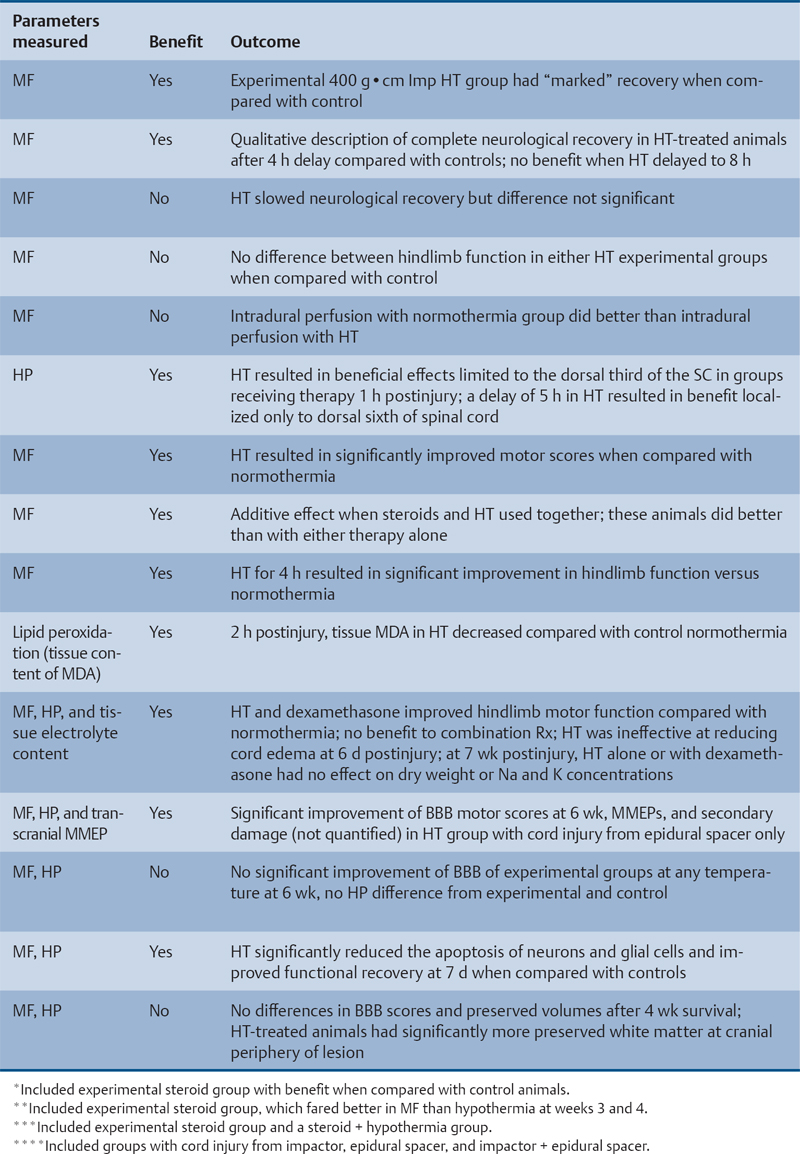
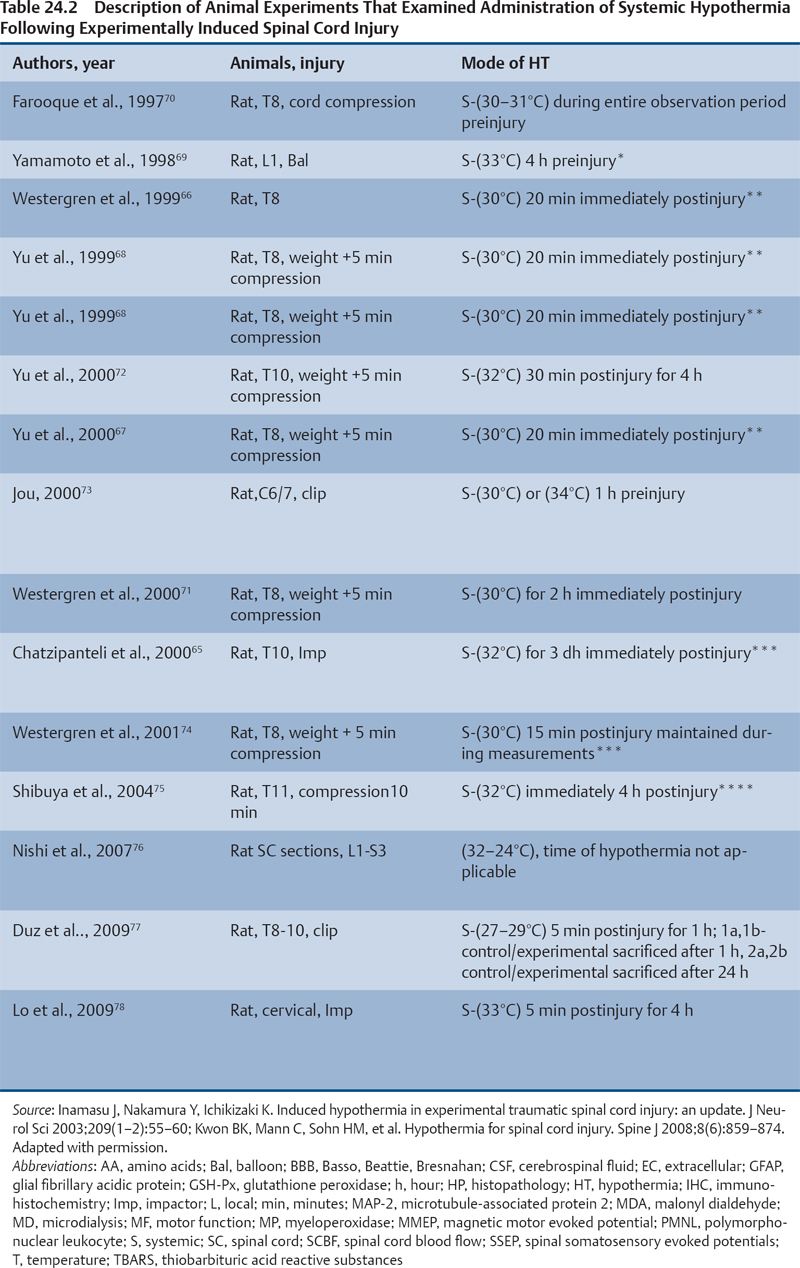
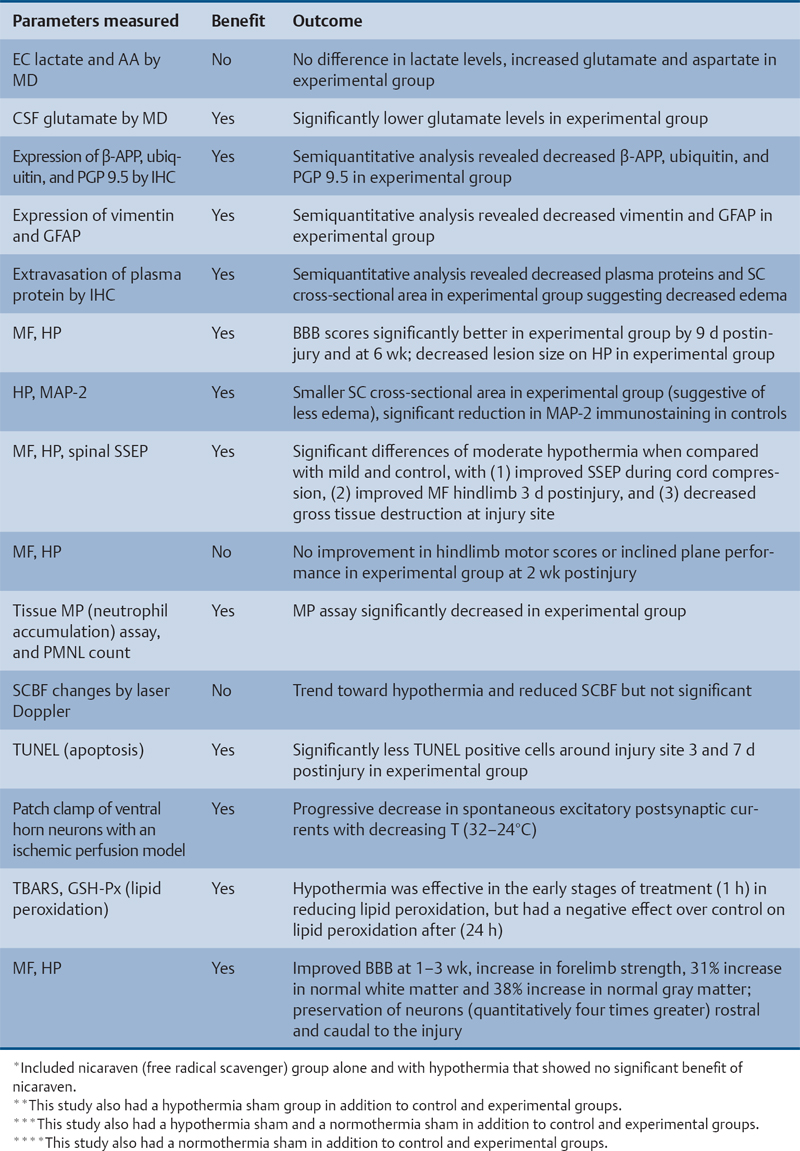
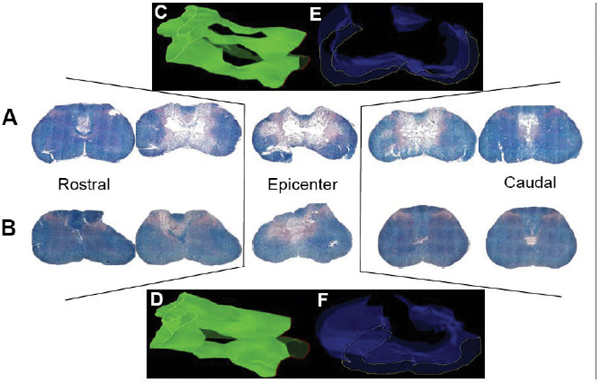
24 Key Points 1. A vast body of literature exists reporting the effects of experimentally induced spinal cord injury in animals and the effects of hypothermia as a neuroprotective agent (Tables 24.1 and 24.2). The two methods of cooling include systemic and local. The results are highly variable, with some studies showing benefit and others not. 2. There have been at least four multicenter, large, randomized trials examining the effects of hypothermia as a neuroprotective agent in TBI, which have either been completed or are ongoing. To date there have been no large, multicenter, randomized, controlled clinical trials examining the benefits and side effects of modest hypothermia following spinal cord injury. 3. Common definitions of hypothermia temperature ranges utilized in both experimental and clinical research include moderate hypothermia (28 to 32°C), modest hypothermia (32 to 34°C), and mild hypothermia (33 to 36°C). 4. There is evidence in various animal models of stroke, epilepsy, TBI, and SCI that supports the statement that hyperthermia is deleterious to the healing nervous system. More than 12,000 persons each year are affected by spinal cord injury (SCI) in the United States (National Spinal Cord Injury Statistical Center). Although several neuroprotective strategies exist for the treatment of SCI, none of them has been established as the standard of care.1 Recent reports in the literature have focused primarily on hypothermia as a treatment following stroke, traumatic brain injury (TBI), cardiac arrest, and intracerebral and aortic aneurysm repair.2–7 Two recent studies found a benefit in mortality and functional outcomes following cardiac arrest.2,8 There also exists a vast history and a relatively recent growing body of literature in both animal and clinical studies examining the effects of hypothermia on the injured spinal cord.9 There is also evidence in various animal models of stroke, epilepsy, TBI, and SCI that hyperthermia is deleterious to the healing nervous system.10–13 The first mention of hypothermia for central nervous system-based pathology in the literature dates back to 1938, when Dr. Temple Fay documented his experience of its use in a cancer patient.14 Fay then followed with a larger study of 120 patients, and reported that surface cooling improved outcomes in TBI. In addition to Dr. Fay, Dr. Rosamoff also pioneered some of the early work in the field of hypothermia for the treatment of TBI.15–17 More recently, ischemic SCI, a common potentially devastating complication following aortic aneurysm surgery, has been an area of interest for cardiovascular surgeons, many having instituted deep hypothermia (18 to 25°C) to prevent this complication. Experimentally induced hypothermia for the treatment of SCI was studied extensively in the 1960s and 1970s. Albin and White were the first to report on the effects of local cooling both intradurally and extradurally on the injured spinal cord in both primate and canine models. Their results indicated a useful beneficial effect of hypothermia in improving neurological outcomes.18–20 Albin and White’s early findings were followed by several other groups, who over the next 10 years utilized various animal models to examine the effects of local hypothermia in SCI (Table 24.1).21–27 However, enthusiasm for pharmacological neuroprotection during the 1980s and 1990s resulted in waning interest in hypothermia as a neuroprotectant for SCI. In the contemporary scientific literature, several groups reported on the positive effects of hypothermia on histopathology or motor function. Green et al. observed histopathological differences between control and hypothermia-treated animals when they analyzed posttraumatic feline spinal cords. These effects were reduced with a delay in the time that hypothermia was begun (i.e., 1 h vs 5 h following injury).22 Wells and Hansebout and Hansebout et al. documented motor outcome improvements in their canine compressive SCI model when hypothermia was administered epidurally for 4 hours either 15 minutes or 4 hours postinjury.21,26 Kuchner and Hansebout found an additive effect when steroids and hypothermia were used together, compared with either therapy alone, in canine motor function.27 These beneficial effects were met by an equal number of groups showing minimal benefit or negative effects of local hypothermia in experimental SCI. Black and Markowitz demonstrated that hypothermia actually slowed neurological recovery, albeit not significantly, in a primate model of SCI when 4 to 8°C saline was placed epidurally for 5 hours following injury.23 Tator and Deecke also showed that primates treated with normothermia and saline perfusion following SCI fared better in motor function than hypothermia-treated groups, whereas Howitt and Turnbull found no difference in hindlimb motor function improvement between rabbits treated with epidural cooling compared with controls.24,28 Epidural saline application following SCI in humans was utilized to a limited extent during the 1960s and 1970s. Most of this early work, although novel, failed to show efficacy due to low sample numbers and the lack of control populations, as well as highly variable time intervals from the actual time of injury until the initiation of cooling.29–36 Perfusion devices used for continuous cooling of the epidural space also required that an acute multilevel posterior surgery be performed. Kwon et al. also listed them in a concise table in a recent review article.14 As also pointed out by Kwon et al. in their recent comprehensive review on hypothermia and SCI, many of these early researchers understood the limitations of these studies, and they were careful to note that no major adverse events occurred.14 Research on hypothermia for various neurological injuries declined in the early 1980s. In the SCI literature, this may have been due to several factors, including (1) the wide variation of results negating or supporting hypothermia in experimental SCI models, (2) the logistical challenges of local hypothermia application and maintenance in open surgery, and (3) complications encountered with profound systemic hypothermia (24 to 33°C) (discussion follows).37 Complications reported with the use of hypothermia include bradycardia, hypothermia, increased risk of deep venous thrombosis (DVT), respiratory infection, lower leukocyte counts, hypotension, coagulopathy, altered drug pharmacokinetics, and skin injury with direct cooling methods.38–43 Of note, many of these adverse events were observed in early studies, where temperatures often ran below 30°C. Hypothermia induces reversible platelet dysfunction and resultant increases in bleeding times as well as disturbances in the coagulation cascades.44,45 Hypothermia-treated patients in the Hypothermia after Cardiac Arrest (HACA) trial developed a higher incidence of bleeding complications, although they were not statistically significant.8 Dysfunction of the immune response under the influence of hypothermia has been reported in both animals and humans.46,47 Russwurm et al., in an in vitro model, were able to link decreased cytokine expression and release, for example, interleukin (IL)-2, in human peripheral blood mononuclear cells. They noted this finding as a potential cause of increased infection risk due to an impaired immune response.48 In the HACA trial, patients in the experimental group had a higher incidence of sepsis and pneumonia (not significant).8 Todd et al. reported a higher incidence of bacteremia in their series on mild intraoperative hypothermia in aneurysm surgery.49 Intravascular devices also pose risks associated with vessel access, including hemorrhage, infection, and thrombosis. Thermoregulatory responses, such as shivering, can also present obstacles to temperature management because this physiological response results in increased metabolic demands, patient discomfort, and elevated temperature. Meperidine-based medical therapies represent the current best methods of treatment of shivering; however, they tend to cause sedation, nausea, and respiratory suppression at high doses.50 A resurgence of interest in the use of therapeutic hypothermia in TBI over the last 15 years has necessitated many scientists to take a “second look” at its potential benefits in SCI.51 A publication by Busto et al. changed the perception that hypothermia could only be effective if administered between 24 and 33°C.52 They found that mild hypothermia (34°C) conferred a protective effect in a rat brain ischemia model. This reinvigorated the modern era of hypothermia research and subsequently led to various animal hypothermia trials in SCI. The manner in which cooling is delivered has also advanced when compared with past methods. Systemic and local cooling are two methods that have been implemented in current animal studies examining the effects of hypothermia in SCI (see Table 24.1 and Table 24.2 for a listing of experimental SCI studies).51 Modern devices such as subcutaneous or epidural heat exchangers have been developed for use in animal models.60 The majority of the animal studies, however, utilize noninvasive external systemic cooling. Rodents, given their smaller body surface areas, can more easily reach target temperatures with systemic external cooling.51 These groups in general have not reported abnormal changes in physiological parameters with hypothermia. This disparity in the reporting of adverse events between animal models and humans in studies of hypothermia could be due to trial design. Most animal studies utilize a lower injury to the thoracic region (e.g., reduces animal care needs), whereas human trials that often include patients with cervical injuries.61 The question of whether systemic temperatures reflect actual temperatures within the spinal cord parenchyma was addressed by Westergren et al.62 They found that in rat models of SCI, esophageal temperatures, although not exact, more accurately reflected intramedullary and epidural temperatures during hypothermia than rectal temperatures. Other methods of experimental temperature monitoring include paravertebral muscle or epidural probes. The physiological effects of hypothermia on the injured spinal cord have been theorized to be due to (1) a decrease in metabolic demand and oxygen consumption, (2) reduction of edema and hemorrhage, and (3) cellular membrane stabilization.51,63,64 Some groups have focused on the histological or neurochemical effects of hypothermia following experimentally induced SCI in animals. Chatzipanteli and colleagues demonstrated attenuation of posttraumatic inflammation measured by decreased myeloperoxidase activity (e.g., neutrophil accumulation) in rats following weight drop and treatment with moderate hypothermia (32°C) for 3 hours when compared with those treated with normothermia.65 Kuchner and colleagues, using a canine balloon compression SCI model, found no differences in electrolyte content or edema in the spinal cords of hypothermia-treated animals versus controls. They did, however, observe improved hindlimb motor function in the hypothermia-treated group (epidural cooling [6°C] begun 15 minutes after injury for 4 hours).55 Yu et al. and Westergren et al. demonstrated an increased number of functional spinal axons and dendrites in a rat weight compression model of SCI when the animals were maintained at 30°C for 2 hours following injury.66,67 Lower ubiquitin and β-amyloid protein precursor (axons) and higher microtubule associated protein 2 (MAP-2) (dendrites) were used as a measure of cellular integrity. This group also confirmed that hypothermia suppressed proliferation of capillaries and astrocytes proximal to injury zones, via reduced vimentin and glial fibrillary acid protein (GFAP), respectively, induced by hypothermia.68 Increased excitatory amino acids (EAAs) (e.g., glutamate, aspartate) and free radicals (e.g., superoxides, nitric oxides) as well as disruption of cell membranes have been shown to occur following experimental SCI and may contribute to secondary injury processes. Two groups, however, have reported conflicting results of the effect of hypothermia on these pathways. In a rat balloon compression model of SCI (L1, 33°C for 4 h preinjury), Yamamoto et al. showed suppression of EAA, whereas Farooque et al. reported an increase in EAA in hypothermia-treated animals (T8, 31°C for the preinjury period).69,70 These differences could potentially be explained by variances in model design and injury level. Tuzgen et al. examined the effect of epidural hypothermia on lipid peroxidation via measurement of the tissue content of malonyl dialdehyde (MDA) in a rat SCI model (C7 clip, 24 to 26°C for 30 min beginning 30 min postinjury) at one time point.54 They found decreased levels of MDA in the hypothermia-treated group yet did not correlate these findings with histological or functional improvements. From 1992 through 2009 there have been five animal studies examining functional recovery following induced SCI and subsequent systemic hypothermia, whereas five have focused on epidural cooling administration. The results in these 10 experimental studies are variable, involving different cooling methods, injury levels, devices, severity, species of animal, and variations in timing, duration, and extent of hypothermia.14 This information was derived from two recent comprehensive reviews.14,51 Morochovic et al., in a rat T8/9 balloon compression model (28.5°C beginning 25 min postinjury for 1 h), reported similar negative results.59 Westergren and colleagues, using a rat weight/compression model (T8), observed no functional benefit to hypothermia (30°C for 2 h following SCI) versus control therapy, whereas Casas et al. also found no functional or histopathological improvement after 6 weeks at four different temperatures 30 minutes postcontusive injury for 3 hours (e.g., normothermic, mild hypothermia 35.3°C, moderate 30.5°C, severe 24.1°C) in their rat T10 injury model.57,71 Yu et al., on the other hand, reported motor function improvement in animals at both 9 days and 6 weeks postinjury (T8, weight-drop contusion, 32°C for 4 h immediately after SCI).72 Specific physiological parameters, such as mean arterial blood pressure (MABP), blood pH, pCO2, and PO2 were well matched at all time points (pre-trauma and 2 or 4 h posttrauma) in both the experimental hypothermia (n = 9) and control groups (n = 7). The normothermia-treated group also showed some recovery of function, suggesting that their model created a partial or subthreshold injury versus prior studies. Functional motor recovery in the control group progressed gradually over a period of 2 to 3 weeks, even though in the first 5 days following injury relatively no movement was noted. After 3 weeks, no further improvement in motor function was observed. In contrast, hypothermia-treated animals demonstrated a continuous improvement in functional scales, such as the Basso, Beattie, Bresnahan (BBB), beginning on day 9, that was significantly better than that of normothermia-treated animals at 6 weeks. The mean area of histopathological damage was also noted to be significantly less in the experimental hypothermia group. Dimar et al., in their rat SCI injury model, created a range of severity of injuries utilizing a compression SCI device, impactor, or both. A local cooling device (T10, 19°C for 2 h immediately after SCI) effectively improved ambulatory function after 5 weeks in the moderate injury group but failed in the other two groups, supporting the idea that there may be certain subthreshold injuries where hypothermia becomes effective.56 Another explanation suggested by Kwon et al. is that regional cooling may be effective for ischemic compression of the spinal cord but somewhat limited when the injury includes a contusive component.14 This statement is also supported by the work of Casas et al., where the authors found no improvement in rat BBB scores at four different temperatures in a contusive SCI model (see earlier discussion).57 Kuchner et al. also found a positive effect of local hypothermia (6°C for 4 h 15 min after injury) that was comparable to postinjury steroid treatment following balloon compression in a canine T13 injury model.55 This study was divided among four groups: (1) dexamethasone-treated (n = 16), (2) local hypothermia (n = 16), (3) dexamethasone + local hypothermia (n = 17), (4) lesion with no therapy (n = 24). A modified Tarlov rating system was used to evaluate clinical motor recovery.79 Jou73 divided 36 rats into three different temperature groups (moderate hypothermia 30°C, mild hypothermia 34°C, normothermia 38°C). Somatosensory evoked potential measurements (SSEPs) were recorded in the upper cervical spine by median nerve stimulation. Gross motor and sensory status of both forelimbs were examined according to a modified scale by Kai et al. 3 days following the injury.80 The animals were then sacrificed. Following a C6/7 clip injury, this group found a significant benefit in hindlimb motor functional recovery (after 3 days) when moderate (30°C) epidural cooling was used for 1 hour preinjury versus mild (34°C) or normothermia. They also observed correlative improvements in histology and SSEPs (e.g., significantly greater preservation of posttraumatic amplitudes noted 60 minutes following injury) in the experimental moderate hypothermia arm.73 This study was important because it introduced an alternate method (i.e., SSEP) of quantifying the effects of hypothermia on the injured spinal cord. Ha and Kim also noted improved functional recovery (at 7 days) when saline was perfused epidurally in a T9 rat impactor SCI model (30°C, postinjury for 48 h).58 Lo and colleagues, using a rat cervical model of contusive SCI, investigated the efficacy of transient hypothermia (beginning 5 minutes postinjury for 4 h, 33°C) for the preservation of neural tissue and improved functional recovery. The experimental group had significant increases in normal-appearing white and gray matter volumes, as well as greater preservation of neurons proximal to the injury epicenter (Fig. 24.1). They observed a faster rate of recovery in open-field locomotor ability (BBB score, weeks 1 to 3) and forelimb strength.78 Over the past few years, technological advances in intravascular and surface cooling delivery have enabled caregivers to more accurately manage this form of treatment.41 Two categories of cooling devices available for human use are external and internal. These devices differ in both their safety and their cooling profiles. External cooling methods have been used more commonly in human trials.41 Methods of external cooling include, in the simplest form, ice packs applied to the body surface, along with bladder and gastric irrigation. More complex external systems include hydrogel pads with continuous feedback mechanisms depending on the patient’s temperature status (Fig. 24.2) (Arctic Sun, Medivance, Inc., Louisville, CO or Cincinnati Subzero Hypothermia System, Cincinnati Subzero Products Inc., OH). Air circulation systems, such as the Bair Hugger (Arizant, Eden Prairie, MN) or TheraKool (Kinetic Concepts, Wareham, England), blow temperature-controlled air across the body surface and are set manually to achieve a core body temperature. Mean time to target temperature, however, for these air-cooling devices in one study was significantly greater (8 h) when compared with other studies that used either intravascular or hydrogel-based systems (1.9–3.5 h).8,81–83 Internal or intravascular cooling devices require that a catheter be inserted into a central vein. These systems utilize a closed system of circulating saline to achieve a particular core temperature. Two devices with recent US Food and Drug Administration (FDA) approval are the Celsius Control System (Innercool Therapies, San Diego, CA) and the Repreive System (Radiant Medical, Redwood, CA). The Coolguard (Fig. 24.3) (Alsius, Irvine, CA) intravascular catheter cooling system was also used in two recent single-center safety trials following SCI.1,84 Newer models contain an internal temperature control system that eliminates the need for placement of rectal or bladder temperature probes. Fig. 24.1 Coronal section series showing improved tissue preservation following hypothermia with three-dimensional (3-D) models of preserved gray and white matter volumes. Hematoxylin & eosin, and luxol fast blue stained 10 mm transverse sections from the injury epicenter, and at 900 and 1500 mm rostral and caudal from a representative spinal cord at 10 weeks after spinal cord injury (SCI) and either (A) normothermia or (B) hypothermia. A significant reduction in the lateral and longitudinal extension of tissue damage can be observed with hypothermia treatment. (C,D) Reconstructed 3-D tissue volumes of normal-appearing gray and (E,F) white matter after normothermia or hypothermia treatment, respectively, using Neuroleucida software, show greater tissue preservation with the application of mild systemic hypothermia following SCI. Scale bar = 1 mm. (Courtesy of Damien Pearse, PhD, University of Miami, Miami Project to Cure Paralysis.)
Hypothermia: Evidence-Based Review
 History of Hypothermia-Based Treatments for Spinal Cord Injury (1940s to 1980s) Controversies in Management
History of Hypothermia-Based Treatments for Spinal Cord Injury (1940s to 1980s) Controversies in Management
Early Animal Models of Hypothermia and Spinal Cord Injury
Early Human Research in Hypothermia and Spinal Cord Injury
 Complications Encountered with Hypothermia
Complications Encountered with Hypothermia
 Resurgence of Mild/Modest Hypothermia
Resurgence of Mild/Modest Hypothermia
 Histopathological and Neurochemical Effects of Hypothermia
Histopathological and Neurochemical Effects of Hypothermia
 Functional Outcomes of Hypothermia in Experimental Models
Functional Outcomes of Hypothermia in Experimental Models
 Human Hypothermia Spinal Cord Injury Trials and Current Cooling Devices
Human Hypothermia Spinal Cord Injury Trials and Current Cooling Devices
Modern Cooling Devices
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


