CHAPTER 20 Humeral head defects—biomechanics, measurements, and treatments
 The surgical management of shoulder instability is very successful, with recurrence rates of less than 5% to 10% with modern techniques and a high level of success returning patients back to their current level of activities.
The surgical management of shoulder instability is very successful, with recurrence rates of less than 5% to 10% with modern techniques and a high level of success returning patients back to their current level of activities. However, if significant humeral head (Hill-Sachs lesion) and/or glenoid lesions are present, the recurrence rate increases tremendously, even after surgical repair, if the bone lesions are not addressed.
However, if significant humeral head (Hill-Sachs lesion) and/or glenoid lesions are present, the recurrence rate increases tremendously, even after surgical repair, if the bone lesions are not addressed. Allograft humeral head reconstruction of Hill-Sachs defects should be considered in patients with large Hill-Sachs lesions, large glenoid lesions, or significant combined lesions that have failed previous anterior soft tissue stabilization and have recurrent instability
Allograft humeral head reconstruction of Hill-Sachs defects should be considered in patients with large Hill-Sachs lesions, large glenoid lesions, or significant combined lesions that have failed previous anterior soft tissue stabilization and have recurrent instability Information supporting anatomic reconstruction has emerged prompting the use of the size-matched allograft reconstruction through either a deltopectoral or direct posterolateral approach as their preferred surgical option.
Information supporting anatomic reconstruction has emerged prompting the use of the size-matched allograft reconstruction through either a deltopectoral or direct posterolateral approach as their preferred surgical option.Introduction
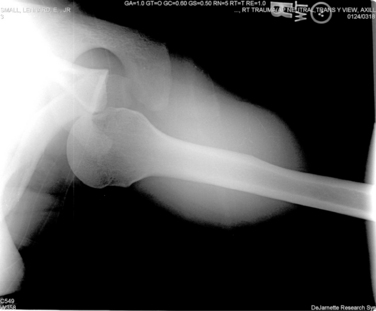
In 1940 Hill and Sachs1 first reported humeral head defects in 74% of recurrent shoulder dislocations. These osseous defects were posterolateral humeral head compression fractures occurring as the humeral head impacts the glenoid rim during the dislocation (Fig. 20-1).
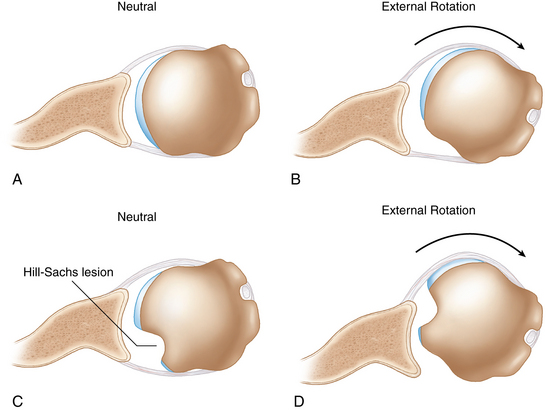
(Modified from Burkhart SS et al: Articular arc length mismatch as a cause of failed Bankart repair. Arthroscopy 16(7):740–744, 2000, Elsevier.)
Despite the prevalence of up to 51% of initial dislocations, Hill-Sachs lesions were historically considered inconsequential with a limited number of surgical treatment options available for their correction.2,3
In the past, the main goal of shoulder instability procedures was maintenance of stable shoulder reduction; hence, many nonanatomic procedures evolved aiming to simply restore glenohumeral stability. These include Bristow or Latarjet coracoid transfer for anterior glenoid defects,4 open capsular shifts,5 rotational proximal humeral osteotomy,6 and “remplissage” infraspinatus transfers for the humeral head defects.7 These procedures yield stability at the expense of motion, but none successfully recreate anatomic joint congruity. Furthermore, all have been associated with long-term complications.8,9
Back in 1984, Rowe et al observed that 76% of shoulders that failed surgical repair for instability had Hill-Sachs lesions. He concluded that Hill-Sachs lesions were the main predisposing factor for postoperative failure.10
Burkhart and DeBeer recognized that patients without bone defects had 4% instability recurrence while those with significant bone defects had 67% recurrence of instability after simple Bankart repairs.11
Furthermore, other studies have shown that large Hill-Sachs lesions resulted in persistent shoulder instability and patient disability.12,13
Biomechanics
Glenohumeral joint stability is derived from the surrounding soft tissues, including the joint capsule, rotator cuff with surrounding musculature, and the osteoarticular surface.14–17
The concavity-compression model as described by Lippitt et al16,17 is an important stabilizing mechanism of the shallow ball-socket glenohumeral joint (Fig. 20-2). It describes the convex humeral head compressed into a concave glenoid-labrum complex by the shoulder girdle musculature as the main mechanism behind shoulder stability. Therefore, loss of humeral head convexity as occurs with large Hill-Sachs lesions could lead to a decrease in this important stabilizing effect. Stehle et al compared the kinematics of the glenohumeral joint and found that in the midrange shoulder position, the soft tissues have minimal function, and the majority of stability is afforded by the osteoarticular surface. With external rotation of the humerus, the surrounding soft tissues contribute significantly to the anterior and coupled superior translation of the humeral head.18
In general, Hill-Sachs defects occupy the posterior and superior positions on the humeral head and can be distinguished from the normal anatomic groove by its more cephalic position within 5 mm of the top of the humeral head.19 The depth and width of the humeral head defect is associated with recurrent shoulder subluxation and dislocation. Ito et al has shown that subluxating and dislocating shoulders have significantly different average Hill-Sachs depths. Overall, the average posterolateral notch depth is 3.9 ± 0.9 mm in the dislocation group and 2.1 ± 1.0 mm in the subluxation group. Furthermore, increasing the number of shoulder subluxations creates shallower lesions. This is attributed to the attrition effect, which is similar to the blunting and wearing away seen with glenoid defects.20
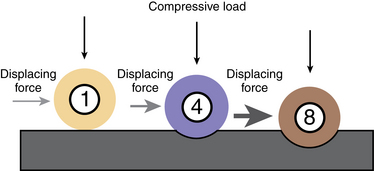
Hill-Sachs lesions effectively reduce the humeral head’s rotational arc length within the glenoid.21 This articular-arc deficit is significant if it causes the lesion to “engage” the anterior rim of the glenoid when the arm is brought into a position of athletic function, defined as 90 degrees of arm abduction and 0 to 135 degrees of external rotation. Hill-Sachs lesions also can lead to the shift of the humeral head within the glenoid. Significant shift occurs when the size of the lesion surpasses 25% of the humeral head diameter. Furthermore, a joint with a large humeral head lesion requires less anterior force and translation to cause the engagement (Fig. 20-3).
Sekiya et al have confirmed that most Hill-Sachs lesions engage the glenoid at 60 degrees of external rotation, which because of scapulothoracic rotation, is equivalent to 90 degrees of shoulder external rotation. Furthermore, the same study has shown that some lesions engage even at 0 degrees of the external rotation. In this study, authors created 4 different size defects (12.5%, 25%, 37.5%, 50% of the humeral head) followed by the osteoarticular allograft repair and evaluated how this changing defect size affected joint stability and anterior translation as defined by the stability ratio (anterior load/compressive load) before dislocation. They concluded that the size and orientation of the defect make important contributions to glenohumeral joint function. Increasing defect size yielded decreased joint stability ratio and required less anterior translation before dislocation, thereby increasing the risk of recurrent instability. In addition, the authors confirmed that osteoarticular allograft repair restored joint stability to the levels of the intact shoulder.22
In another study, Sekiya et al evaluated the biomechanical effect of a 25% Hill-Sachs defect at three clinically relevant glenohumeral angles as compared with the intact shoulder in response to a loading pattern consisting of a compressive load and either an anterior or posterior load (Fig. 20-4). They evaluated glenohumeral translations, capsular in situ forces, and joint contact forces and concluded that an isolated 25% Hill-Sachs lesion is not a risk factor for recurrent shoulder instability if a perfect capsular repair is performed and allowed to heal. However, they cautioned that the Hill-Sachs lesions occupying 25% of the humeral head may not be the problem in isolation, but should be a marker of a more severe and higher energy injury with concomitant associated structural damage to glenoid or rotator cuff. Furthermore, they recommended diligent surgical technique for capsular repairs and rehabilitation in the setting of present bone defects because the margin of error is significantly smaller when bony lesions are present.23
Along with previously described soft tissue procedures for the treatment of these consequential Hill-Sachs lesions, new surgical techniques have recently emerged that attempt to directly repair the humeral head osseous defects and restore normal anatomy. One technique that has been described is percutaneous transhumeral head plasty. However, it has limited utility because of its inherent surgical complexity.24,25 Furthermore, reconstructive procedures like shoulder resurfacing or replacement surgery remain inappropriate for young athletic patients seeking to return to a preinjury level of sport participation.
Several authors believe that the humeral head allograft reconstruction is the best technique to fully recreate normal humeral head anatomy and reestablish joint stability. Furthermore, recurrent glenohumeral instability secondary to either a humeral head or glenoid bone defect should be addressed with surgical allograft reconstruction.26–29 In this chapter we highlight two technical variations for the humeral head allograft reconstruction and defer glenoid reconstruction to be covered in chapters 16–19.
Biomechanics Pearls
Indications/contraindications
The first surgical goal of the humeral head osteochondral allograft reconstruction is to identify the patients who would benefit from this reconstruction. This would prevent the repetitive trauma created by the recurrent dislocations and circumvent the need for subsequent revision surgery. However, patients with large Hill-Sachs lesions that have failed previous anterior soft tissue stabilization and have recurrent instability also should be considered for surgical allograft reconstruction.
Indications
Preoperative history, examination, and radiographic findings
Preoperative history
It is important to elicit information about the factors that may increase the propensity for recurrence of instability such as history of seizures, gait abnormalities resulting in recurrent falls, drug and alcohol dependence, and the patient’s expected future activities (sport, work).
Radiographic findings
West Point view is performed as described by Rokous et al and represents a modified prone axillary view performed with the humerus abducted 90 degrees from the trunk and the cassette placed cephalad to the shoulder and perpendicular to the table.30 The hand and arm are kept pronated so that the anterior surface of the humeral shaft faces the floor. The roentgen beam is directed at the axilla and is angled 25 degrees medially and 25 degrees cephalad, and is centered inferior and medial to the acromioclavicular joint.
Evaluating shoulders with recurrent anterior dislocation,31 Rozing et al compared six radiographic views for detection of the Hill-Sachs defect and found that the Hill-Sachs defect was most often identified by the Stryker notch view.31 Similarly, Pavlov et al demonstrated a 92% diagnostic yield when evaluating the Hill-Sachs defects using the internal rotation and Stryker notch views.32
In their original article, Hill and Sachs found the defect in 74% of their patients with recurrent anterior dislocations while Resnick et al found Hill-Sachs lesions in 15 out of 15 recurrent dislocators using these modified views.33
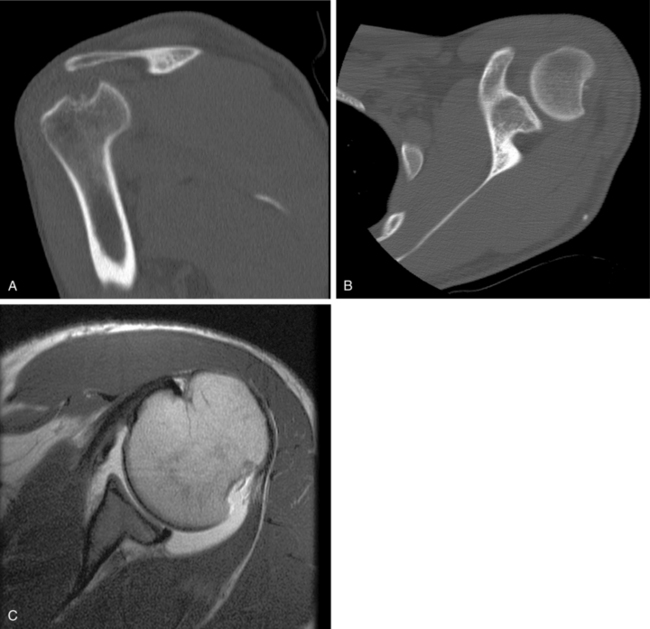
Computed tomography (CT) should be included in the evaluation of patients with osseous glenohumeral defects because plain radiographs often underestimate the size of the Hill-Sachs defect. The multiplanar CT scan with three-dimensional reconstruction is often necessary to fully appreciate the extent of the osseous lesion (Fig. 20-5). Accurate CT scanning also is essential for the preoperative templating measurements necessary to select size-matched humeral allografts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree