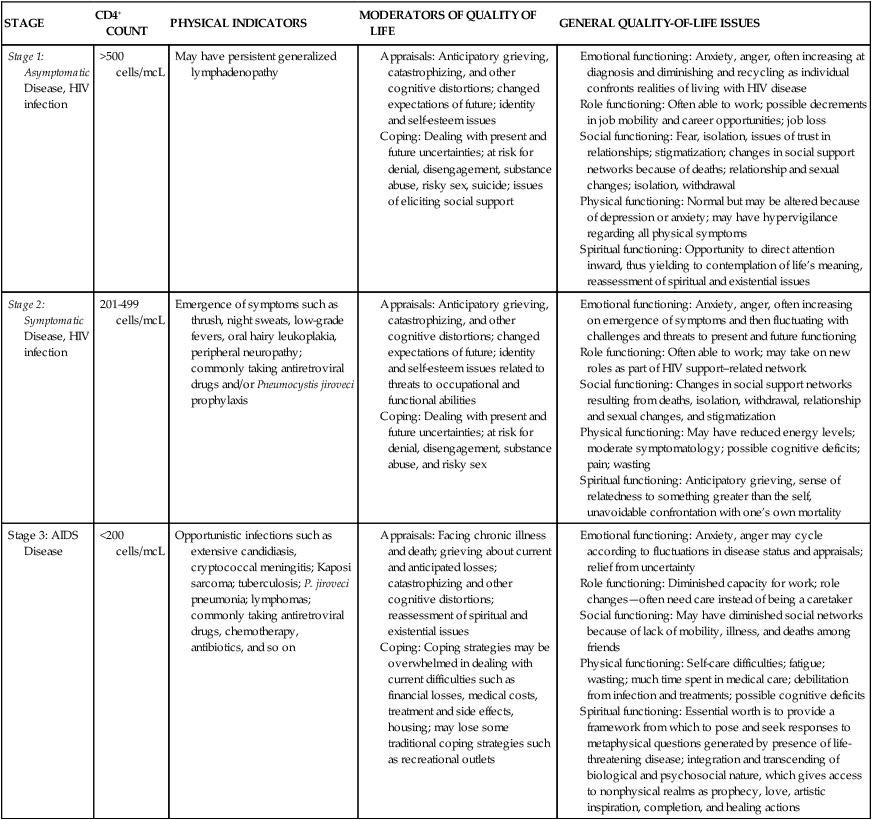
KERRI SOWERS, PT, DPT, MARY LOU GALANTINO, PT, PhD, MSCE and DAVID M. KIETRYS, PT, PhD, OCS After reading this chapter the student or therapist will be able to: 1. Appreciate the role of the immune system in chronic HIV disease. 2. Discuss the neuropathological features of HIV infection and understand potential neurocognitive and neuropsychological alterations that may occur. 3. Understand the various systems (integumentary, musculoskeletal, cardiopulmonary, and neurological) that affect function in HIV-infected adult and pediatric patients. 4. Appreciate the role of psychoneuroimmunology in HIV rehabilitation management. 5. Establish safe exercise parameters in the HIV-positive population. Initially recognized in 1982, acquired immunodeficiency syndrome (AIDS) has been one of the leading causes of death among young adults in the United States since that time. Even with significant advancements in the medical management of the disease, there continues to be a devastating impact in the developing world.1,2 The course of human immunodeficiency virus (HIV) disease in industrialized nations, including the United States, has changed dramatically as a result of advancements in medications used to treat the disease, as well as increased public awareness and the expansion of programs in poverty-stricken areas. Although the disease was once considered a death sentence, the long-term prognosis for those diagnosed with HIV/AIDS has drastically changed in most industrialized countries. In the United States, HIV is no longer found in the top 10 causes of death for the entire adult population. Yet, HIV is the seventh leading cause of death in young adults (age 20 to 24 years); for both older teens (ages 15 to 19 years) and young teens (ages 10 to 14 years), HIV is ranked fourteenth; for children (ages 5 to 9 years), HIV drops to nineteenth on the list.3 Although still alarming, this is actually an improvement compared with the 1990s. Most epidemiologists and clinicians attribute improved life expectancy to the impact of new, highly active antiretroviral therapies (HAART). Implementation of these medications has resulted in a decline in AIDS deaths nationally.4,5 However, the incidence of HIV disease, which demonstrated some decrease in the 1990s, demonstrated an increase from 1999 to 2006. HAART regimens have fostered longevity for many, resulting in the evolution of HIV infection into a chronic disease. Individuals previously disabled by the disease now have the potential to return to work and functional activities and often can expect to live a normal life expectancy. Despite these gains, HIV disease, related comorbidities, and the side effects of medications used to treat the disease have a great impact on rehabilitative medicine because of the multisystem involvement, which often progresses slowly throughout the life span. The advancements in medications that have led to increased life expectancies and improved functional capabilities have also led to a greater demand for rehabilitative services. HAART has slowed and prevented the progression from HIV infection to AIDS and from AIDS to death.6 In communities with access to antiretroviral medications, the incidence of perinatally acquired AIDS has declined significantly as a result of administration of HAART during pregnancy.7 Unfortunately, perinatal transmission of the virus in developing nations continues to be a crisis. In 2008 the Centers for Disease Control and Prevention (CDC) revised its definition of AIDS and its classification system of HIV disease. To reflect current scientific knowledge, the new system elucidates the importance of CD4+ T-lymphocyte cell counts as indicators for pharmacological disease management. Based on laboratory criteria and clinical presentation, the disease is classified into four stages. Stage 1 has no AIDS-defining condition and either a CD4+ T-lymphocyte count greater than or equal to 500 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes greater than or equal to 29%. Stage 2 also has no AIDS-defining condition and either a CD4+ T-lymphocyte count of 200 to 499 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes of 14% to 28%. Stage 3 is classified by the CDC as AIDS; it is defined as a CD4+ T-lymphocyte count less than 200 cells/mcL or a ratio of CD4+ T-lymphocytes to total lymphocytes less than 14% or documentation of an AIDS-defining condition (Box 31-1). A fourth stage was also identified as HIV Infection, Stage Unknown (for cases in which no information is obtained regarding the CD4+ T-lymphocyte counts or ratios or regarding any AIDS-defining conditions); the primary use of this stage is for surveillance purposes.8 The entire spectrum of illness from initial diagnosis to AIDS can be covered by the term HIV disease. In addition, the terms acute HIV infection, asymptomatic HIV disease, symptomatic HIV disease, and advanced HIV disease (AIDS) are used throughout this chapter. In general, asymptomatic HIV disease corresponds with Stage 1, symptomatic HIV disease with Stage 2, and advanced HIV disease (AIDS) with Stage 3. Table 31-1 presents the various modifiers of quality of life throughout the various stages of HIV disease. TABLE 31-1 QUALITY-OF-LIFE ISSUES FOR HIV DISEASE STAGES It is currently estimated that over 30 million people are infected with HIV globally. In the United States, the CDC estimated that 1.1 million adults and adolescents were HIV positive at the end of 2006. Because of complex social and economic factors, African Americans are disproportionally affected, with approximately half of the cases in the United States involving this minority group. Alarmingly, it is estimated that approximately 25% of individuals in the United States infected with HIV are unaware of the infection.9 In the most recent publication of the World Health Report from the World Health Organization (WHO), HIV/AIDS is the sixth leading cause of death worldwide, with an estimated 2.04 million deaths per year.10 Worldwide, of the 33 million people (all ages) living with HIV, 30.8 million are adults, 15.5 million are women, and 2.0 million are children (under the age of 15 years). New HIV infections in 2007 totaled 2.7 million, with 2.3 million in adults and 370,000 in children under 15 years old. Global AIDS deaths totaled nearly 2.0 million; adult deaths were 1.8 million, whereas children under age 15 years totaled 270,000.11 It was estimated that in 2006 the United States had approximately 14,561 deaths from AIDS-related illnesses.9 Tuberculosis (TB) is a former leading microbial killer; it is caused by infectious bacteria that spread through the air in microscopic droplets. WHO estimates that there were 9.27 million new TB cases in 2007; of those, 1.37 million cases (14.8%) were in HIV-positive individuals. Approximately 456,000 deaths caused by TB occurred in HIV-infected individuals (23% of the estimated 2 million HIV deaths were caused by TB).12 The immune system is complex and dynamic, comprising a multitude of components and subsystems, all of which interact continuously. The normal immune system has two main components, or lines of defense, against illness (Figure 31-1). The first is the innate, or inborn, component, which includes the skin, the cilia and mucosal linings of the respiratory and digestive systems, the gastric fluids and enzymes of the stomach, and the phagocyte cells. This innate component of the immune system keeps pathogens out of the body by creating barriers against them, by ejecting them, or by enveloping them and eliminating them. The second, the acquired component of the immune system develops defenses against specific pathogens, starts in utero, and continues throughout life. It is acquired (or antibody) immunity that is most pertinent to understanding HIV infection and its progression. Acquired immunity is divided into humoral and cell-mediated responses. Humoral immunity depends on the production of antibodies. This response is effective for disposing of free-floating or cell-surface pathogens. The cell-mediated response is required to destroy infected cells, those with intracellular pathogens. Cell-mediated immunity is essential for destroying pathogens responsible for the opportunistic infections and neoplasms that are associated with AIDS.13,14 For the study of HIV pathology, it is important to consider three types of immune system cells: macrophages, T lymphocytes (T cells), and B lymphocytes (B cells). Macrophages originate in the bone marrow and then migrate to the organs in the lymphatic system. Macrophages recognize and then phagocytize antigens—substances deemed foreign to the body. All but a fragment of the antigen is digested by the macrophage. This remaining fragment protrudes from the cellular surface, where it is then recognized by T and B cells, allowing those cells to develop an appropriate immune response.15 Both of the lymphocytes (T and B cells) originate in the bone marrow. Their differentiation into T and B cells depends on where they develop immunocompetence. Immunocompetence is the ability of the immune system to mobilize in response to an antigen; it can be weakened secondary to age-related changes, radiation therapy, chemotherapy, or viral infections. T cells migrate to the thymus to develop this ability. B cells develop it before leaving the bone marrow. T cells travel to lymph nodes, the spleen, and connective tissues, where they wait to phagocytize the antigens in the manner previously described. B cells function in the same way against free-floating blood-borne pathogens.15 In the process of identifying and destroying antigens, the acquired immune system retains a memory of the antigen. This allows the immune system to respond more rapidly and effectively to the pathogen if it is reintroduced into the body. Herein lies the pertinence of vaccination and the phenomenon of being immune to an illness.15 HIV belongs to a class of viruses known as retroviruses, which carry their genetic material in the form of ribonucleic acid (RNA) rather than deoxyribonucleic acid (DNA). HIV primarily infects the mononuclear cells, especially CD4 and macrophages, but B cells are also infected.16 HIV binds to the receptor sites on the surface of the CD4 lymphocytes, eventually fusing with and then entering the cells. Reverse transcriptase released from the HIV allows a DNA copy of the virus to be made within the host cell, which then becomes integrated into the host cell genome. Other enzymes, such as integrase and protease, turn the lymphocyte into a “virus factory,” and replicated virions bud out of the cell to infect others. When the CD4 cell count drops below 200 cells/mcL, the individual is diagnosed with an opportunistic infection or other AIDS-defining illness, or the individual demonstrates wasting syndrome or HIV-related dementia, he or she is reclassified as being in Stage 3—advanced HIV disease or AIDS. It is possible for patients in this stage to demonstrate remarkable recovery in terms of both laboratory values and function with HAART. Individuals who do not have access to HAART, or individuals in whom HAART has failed, will eventually die as a result of the effects of opportunistic infections that inevitably occur. Quality-of-life issues throughout the stages of HIV disease are described in Table 31-1. For the healthy HIV-negative adult, the average CD4 cell count is approximately 1000 cells/mcL. However, counts fluctuate over time and may range from 500 to 1600 cells/mcL.17 A CD4 cell count of 200 cells/mcL marks a critical point in the course of HIV infection, often indicating that the stage of advanced HIV infection or AIDS has been reached. Serious opportunistic infections are likely to occur once this level of immune depletion has been attained.18–20 Exercise, stress, seasons of the year, serum cortisol level, and the presence of acute or chronic illness and infection have all been reported to affect CD4 cell counts. Thus the initial CD4 lymphocyte numbers should be confirmed by repeat testing. Caution should be exercised to avoid overinterpreting small changes in CD4 lymphocyte test results. The overall trend of CD4 counts is more important than any single value. Testing is typically done at a frequency of four times annually. In addition to CD4 cell counts, CD4/CD8 ratios are used to evaluate the status of the immune system. CD4 counts above 500 cells/mcL indicate no need for antiretroviral therapy because individuals are generally asymptomatic. It is currently recommended that HAART be initiated when CD4 levels are below 350 cells/mcL, with individual parameters influencing the decision.21 CD4 cell counts below 200 cells/mcL are an indication for prophylactic Pneumocystis jirovechi (previously referred to in the literature as Pneumocystis carinii; this text will refer to the current terminology) pneumonia (PCP) and toxoplasmosis measures. Persons with counts below 100 cells/mcL may also receive prophylactic agents against cytomegalovirus (CMV) infection, infection with Mycobacterium avium complex (MAC), and fungal infections such as cryptococcosis and candidiasis.13 In addition, it is recommended that HIV-positive pregnant women, those with HIV-associated nephropathy, and those co-infected with the hepatitis B virus be started on a HAART regimen immediately.22 Table 31-2 is a summary of common pharmacological agents prescribed to combat opportunistic infections and, most pertinent to rehabilitation, their potential side effects. TABLE 31-2 Black Box warning: Buildup of acid in the blood (has been fatal in pregnant women when combined with Videx/Videx EC); fatty liver; damage to the pancreas (when combined with Videx/Videx EC). Numbness, tingling, or pain in the hands or feet (peripheral neuropathy); lipodystrophy; muscular weakness (rare); increased cholesterol and increased triglycerides. Testing for the amount of HIV in plasma by measuring viral RNA has become a standard component of the management of HIV-infected patients.23 There are important prognostic implications for the amount of viral load in persons with HIV disease.24 In patients with higher viral loads, disease progression is more rapid, both immunologically, in terms of the rate of CD4 cell count decline, and clinically, in terms of development of AIDS-defining illness. In addition, the plasma levels in HIV-positive pregnant women directly correlate with the risk of perinatal transmission.25 Viral load is an important useful marker for judging the effectiveness of various antiretroviral drug interventions.26,27 There are several assays available for testing HIV for resistance to antiretroviral agents. Genotype or phenotype testing is used to determine whether the virus has mutated. The results of genotype or phenotype testing provide important information about resistance to specific antiretroviral drugs. If a mutant form is resistant to a particular antiretroviral drug, the HAART regimen may be altered so that the potential for viral suppression is maximized. Changes in the drug combinations used for HAART to respond to viral resistance are referred to as salvage therapy. Like genotypic testing, phenotypic testing may not detect small subpopulations of resistant HIV.28 Researchers continue to work on developing effective HAART components and vaccines. The primary goal of antiretroviral therapy is to achieve prolonged suppression of HIV replication.23,29 At this time, there are six classes of HIV medications. Two classes of drugs, receptor site inhibitors and fusion inhibitors (FIs), work to prevent HIV from successfully entering the cell. CCR5 inhibitors (CIs) include maraviroc (Selzentry), a CCR5 co-receptor antagonist (receptor site inhibitor). Receptor site inhibitors are the most recently approved class of drugs. FIs such as enfuvirtide (Fuzeon) or T-20 act outside the T cells by blocking the entry of HIV into the cell. T-20 is often used as part of salvage therapy; it is a twice-daily injectable drug with a cost of more than $25,000 per year. The four other classes of drugs work within the cell by interfering with one of three enzymes that are involved with the replication process: reverse transcriptase, integrase, and protease. These classes include nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors (INIs), and protease inhibitors (PIs). In 1987, zidovudine (AZT), an NRTI, was first approved by the U.S. Food and Drug Administration. Since that time, several more NRTI drugs have been approved.30 Other drugs, such as nevirapine and efavirenz, also inhibit the reverse transcriptase enzyme, but they are not nucleoside analogs. These NNRTIs bind to the enzymatic binding pocket of the reverse transcriptase gene and block binding by the nucleosides.31 Like reverse transcriptase, integrase is an enzyme that is active in the early stages of the replication process, and INIs can be used to interrupt its function by preventing the integration of the virus in the host cell’s DNA.32 INIs, such as elvitegravir or raltegravir (Isentress), are one of the most recently approved classes of drugs. Another drug target for anti-HIV agents is the protease enzyme. The PI drugs are structurally different from other drugs and include agents such as ritonavir, indinavir, nelfinavir, and saquinavir.33 HAART may be NNRTI or PI based (i.e., NNRTI and PI drugs are used in combination with an NRTI such as AZT). There has been a gradual evolution of pharmacology that has allowed for multiple drugs to be combined into one pill. Thus the number of pills required per day as well as the administration schedule have become increasingly more manageable over recent years. However, drugs from different classes (NRTI, NNRTI, and PI) are typically included in HAART. The current recommendation from the Department of Health and Human Services for a treatment-naïve patient is either one NNRTI and two NRTIs or a PI (boosted with ritonavir) and two NRTIs.22 Because of the rapidly evolving nature of HAART, the reader is advised to consult with the CDC for the most current clinical practice guidelines. Current medication regimens can significantly reduce the HIV level not only in the peripheral blood but also in the lymphoid tissue and the central nervous system (CNS).34 The goal of HAART is to reduce HIV viral load to undetectable levels in serum. The greatest challenge with HAART is resistance to one drug in a class of agents, which may induce partial or complete resistance with other agents, depending on the specific mutations involved.28,35 In a field that is rapidly changing, specific recommendations for antiretroviral therapy are best made by an infectious disease specialist with experience in the management of patients with HIV disease. The major therapeutic decisions include (1) when to initiate therapy, (2) what drugs to prescribe, (3) when to change therapy, and (4) which drugs to change to. When PIs were introduced as a complement to already existing NRTI and NNRTI drugs, the mortality rate of HIV-infected patients and the incidence of opportunistic infections decreased, most likely as a result of the increased use of combination HAART.36 The role of drugs with immunomodulating activity in combination with HAART is also undergoing extensive research.37,38 Drug regimens for HIV disease are dynamic, and clinical practice guidelines are consistently updated; many changes in the approach to drug interventions can be expected as HIV infection continues to be a chronic disease.39 HIV-positive individuals respond less well than do uninfected persons to most vaccines. The degree of immunodeficiency present at the time of vaccination has an impact on the response to hepatitis A or B, pneumococcal, and influenza A and B vaccines.40 Patients with a CD4 count of more than 200 cells/mcL have a more successful response to the vaccine. Patients should be informed that the extent and duration of the protective efficacy of these vaccines are still uncertain. Vaccination for HIV has the potential to prevent or control disease progression. The development of an effective preventative vaccine for HIV is an area of continuing research. The first human immunizations with the potential AIDS vaccine took place in 1986 in healthy seropositive volunteers in France and Zaire. Low levels of both humoral and cell-mediated immune responses resulted. One conclusion of this study is that booster vaccinations could be effective.41 Several vaccine candidates have been developed and tested in human phase 1 or 2 trials. To date, at least 13 vaccine candidates have been created with use of different forms of recombinant proteins that target the HIV envelope. Research has found that the vaccine candidates introduced antibodies that rarely neutralized HIV progression, as evidenced by assessment of patient blood counts (i.e., CD4 counts). Furthermore, these recombinant proteins rarely produced a cellular response that would target and destroy cells already infected with HIV.42 Currently there is no evidence of a vaccine that produces extended, high-titer neutralization across a variety of HIV strains.42 The most recent clinic trial, the Thai Phase III HIV vaccine trial (also known as RV 144), was completed in September 2009. The study incorporated two vaccines, a prime vaccine (ALVAC-HIV) and a booster vaccine (AIDSVAX B/E), which were based on strains found in Thailand, where the clinical trial was conducted. The clinical trial involved over 16,000 volunteers who received either the vaccine combination or a placebo. The clinical trial found that the vaccine regimen was safe and modestly effective, demonstrating that the vaccine combination lowered the HIV infection rate by 31.2% compared with the placebo. The study also found that the vaccine had no effect on the viral load of those volunteers who became infected during the clinical trial.43 Genetic mutation of the virus further complicates attempts to disable it. Genetically similar but distinguishable strains of HIV can exist in one individual. Furthermore, drug-resistant strains of HIV have been identified.44 Yet another difficulty with vaccination development is a lack of animal models. Chimpanzees replicate simian immunodeficiency virus, a similar but not identical disease. In addition, an average of 12 years and $231 million is required for a new drug to gain Food and Drug Administration approval. Many major pharmaceutical companies seem wary of the immense research expenses and potential liability risks linked to vaccine development. The result is that smaller biotechnology companies with fewer resources are assailing the complicated problems of HIV infection.13 Recent advancements and plans for future clinical trials are largely supported by military or government programs. Researchers are optimistic that the vaccine will induce both humoral and cellular immune responses and have no toxic effects. It will protect against initial infection and retard disease onset in infected individuals.
Human immunodeficiency virus infection: living with a chronic illness
Identification of the clinical problem

Epidemiology
Normal immunity
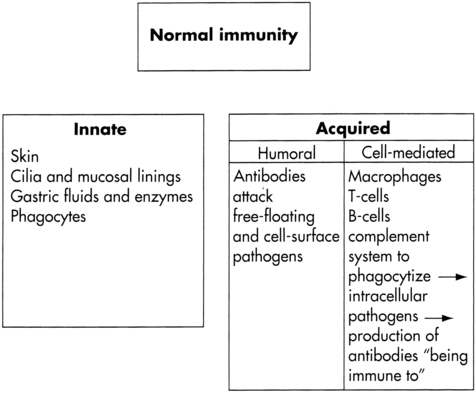
 Main components of immunity.
Main components of immunity.
Acquired immunity
Pathogenesis of HIV disease
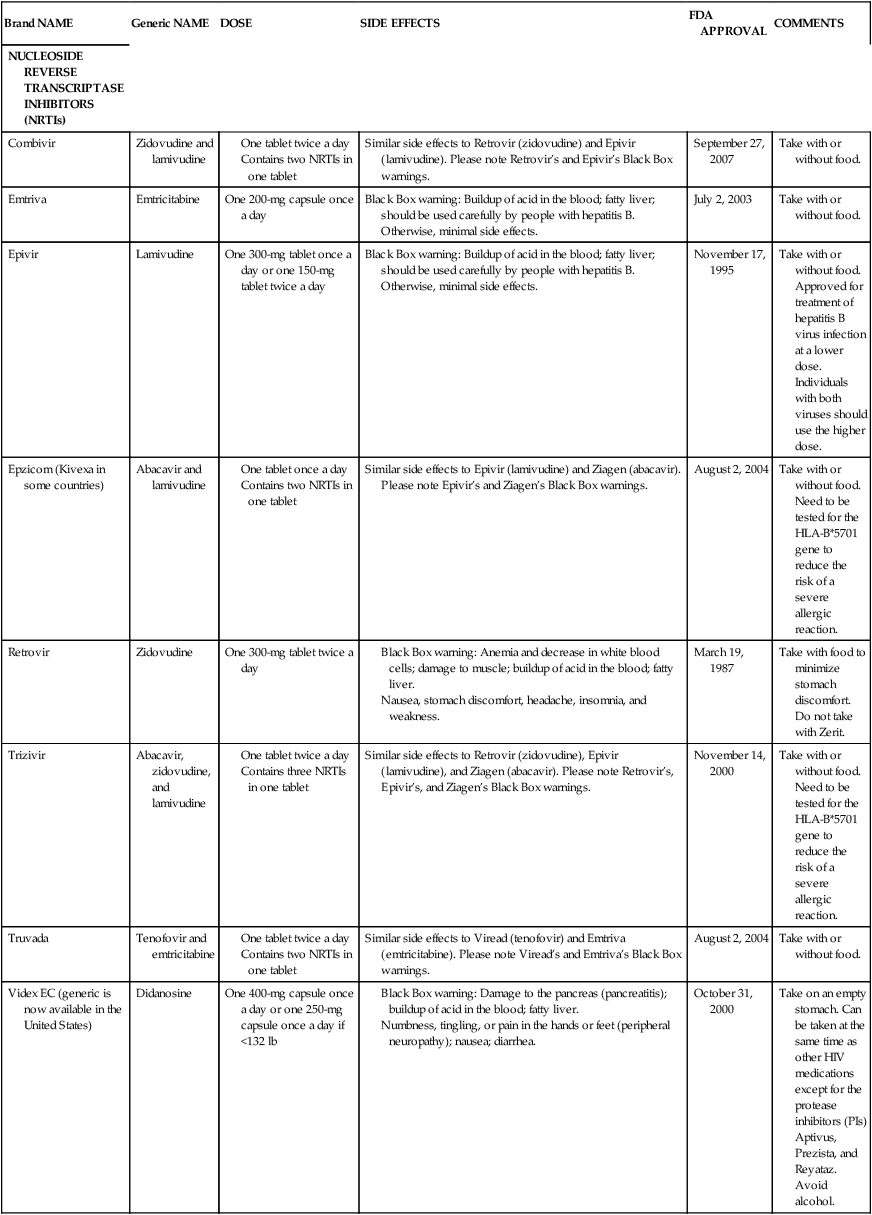
Medical management
Cell counts and prophylaxis

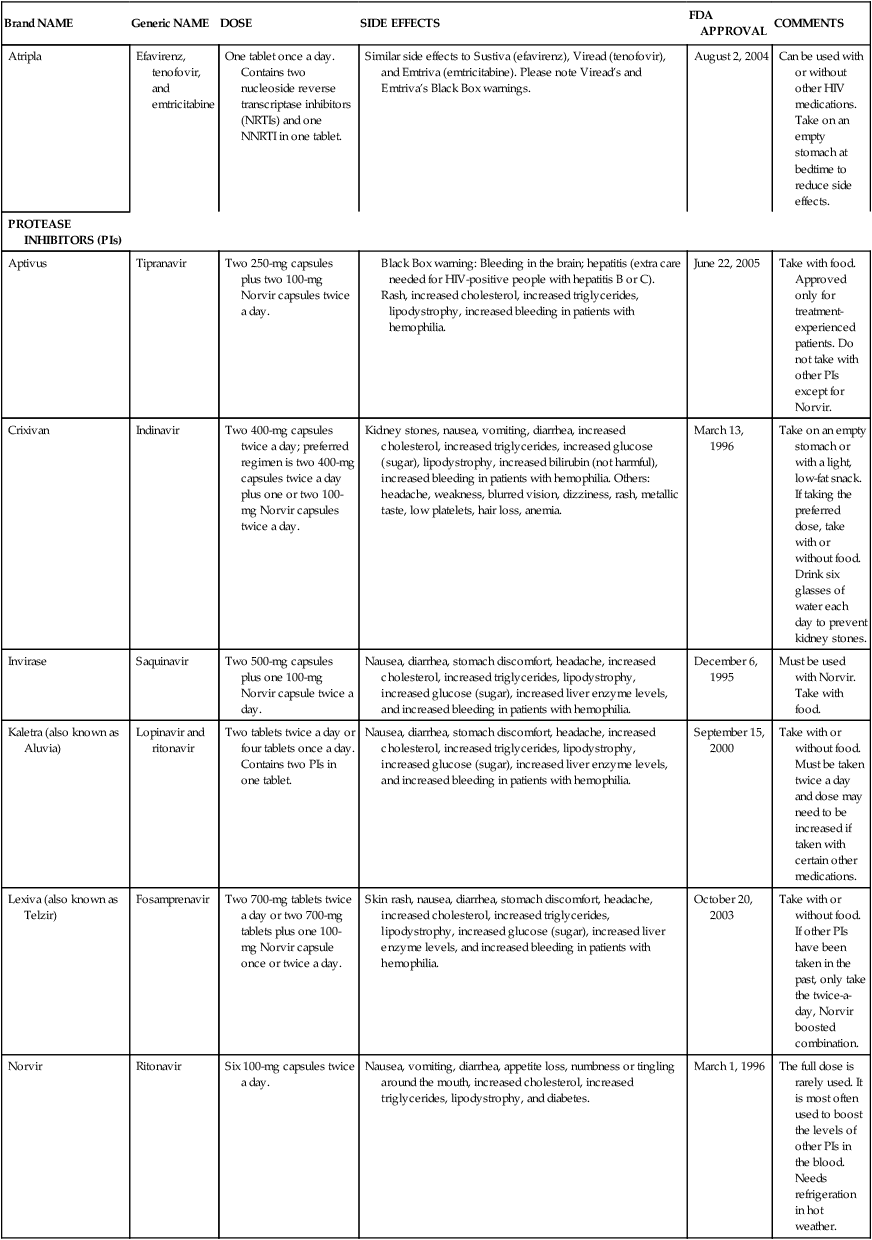
Brand NAME
Generic NAME
DOSE
SIDE EFFECTS
FDA APPROVAL
COMMENTS
NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS (NRTIs)
Combivir
Zidovudine and lamivudine
Similar side effects to Retrovir (zidovudine) and Epivir (lamivudine). Please note Retrovir’s and Epivir’s Black Box warnings.
September 27, 2007
Take with or without food.
Emtriva
Emtricitabine
One 200-mg capsule once a day
Black Box warning: Buildup of acid in the blood; fatty liver; should be used carefully by people with hepatitis B. Otherwise, minimal side effects.
July 2, 2003
Take with or without food.
Epivir
Lamivudine
One 300-mg tablet once a day or one 150-mg tablet twice a day
Black Box warning: Buildup of acid in the blood; fatty liver; should be used carefully by people with hepatitis B. Otherwise, minimal side effects.
November 17, 1995
Take with or without food. Approved for treatment of hepatitis B virus infection at a lower dose. Individuals with both viruses should use the higher dose.
Epzicom (Kivexa in some countries)
Abacavir and lamivudine
Similar side effects to Epivir (lamivudine) and Ziagen (abacavir). Please note Epivir’s and Ziagen’s Black Box warnings.
August 2, 2004
Take with or without food. Need to be tested for the HLA-B*5701 gene to reduce the risk of a severe allergic reaction.
Retrovir
Zidovudine
One 300-mg tablet twice a day
March 19, 1987
Take with food to minimize stomach discomfort. Do not take with Zerit.
Trizivir
Abacavir, zidovudine, and lamivudine
Similar side effects to Retrovir (zidovudine), Epivir (lamivudine), and Ziagen (abacavir). Please note Retrovir’s, Epivir’s, and Ziagen’s Black Box warnings.
November 14, 2000
Take with or without food. Need to be tested for the HLA-B*5701 gene to reduce the risk of a severe allergic reaction.
Truvada
Tenofovir and emtricitabine
Similar side effects to Viread (tenofovir) and Emtriva (emtricitabine). Please note Viread’s and Emtriva’s Black Box warnings.
August 2, 2004
Take with or without food.
Videx EC (generic is now available in the United States)
Didanosine
One 400-mg capsule once a day or one 250-mg capsule once a day if <132 lb
October 31, 2000
Take on an empty stomach. Can be taken at the same time as other HIV medications except for the protease inhibitors (PIs) Aptivus, Prezista, and Reyataz. Avoid alcohol.
Viread
Tenofovir
One 300-mg tablet once a day
October 26, 2001
Take with or without food. Approved for treatment of the hepatitis B virus. Can raise Videx EC level in the blood and increase side effects.
Zerit
Stavudine
One 40-mg capsule twice a day or one 30-mg capsule if <132 lb
June 24, 1994
Take with or without food. Do not take with Retrovir or Combivir.
Ziagen
Abacavir
One or two 300-mg tablets once a day
Black Box warning: Severe allergic reactions (symptoms include fever; rash; severe nausea, diarrhea, abdominal pain; sore throat; cough; and shortness of breath); buildup of acid in the blood; fatty liver.
December 17, 1998
Take with or without food. Need to be tested for the HLA-B*5701 gene to reduce the risk of a severe allergic reaction.
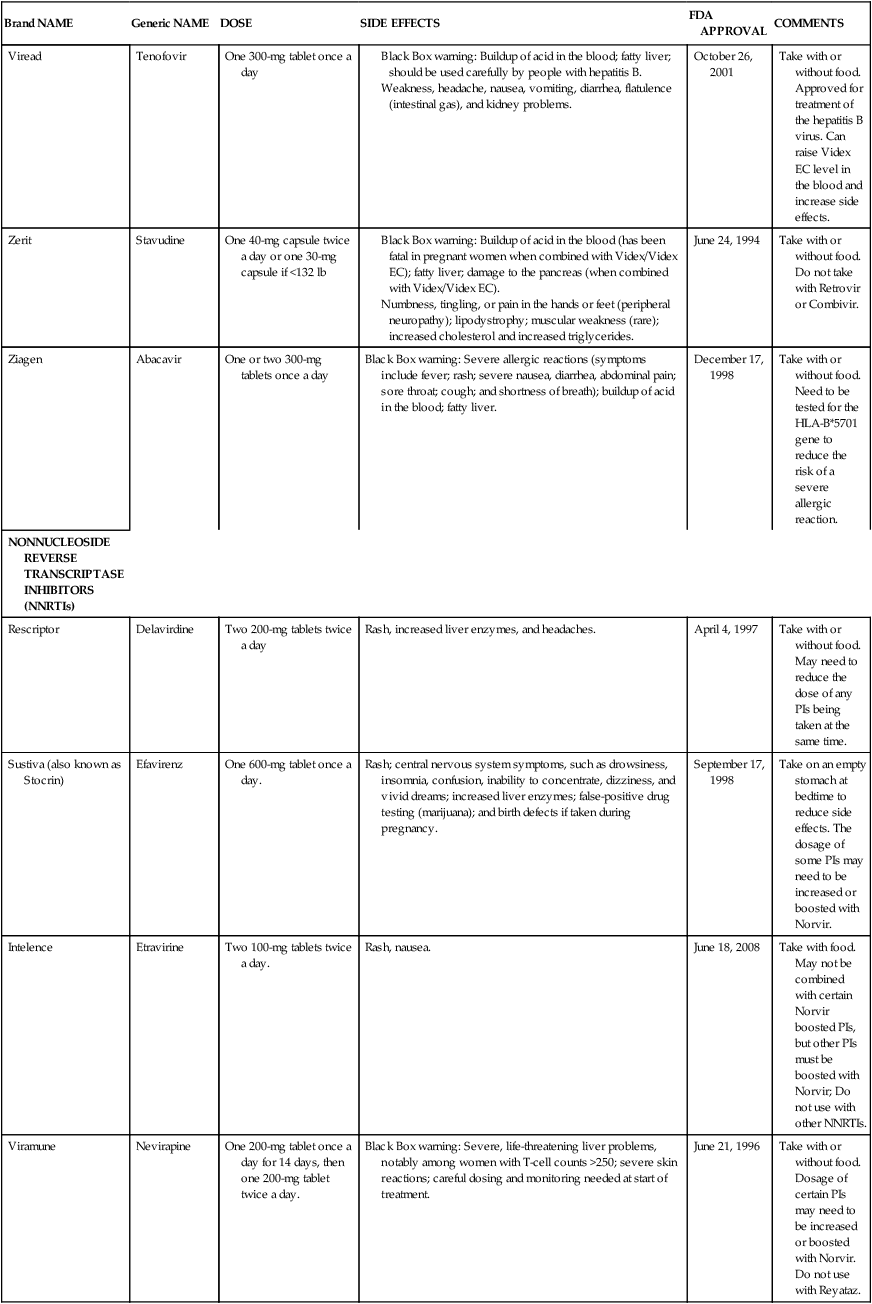
NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS (NNRTIs)
Rescriptor
Delavirdine
Two 200-mg tablets twice a day
Rash, increased liver enzymes, and headaches.
April 4, 1997
Take with or without food. May need to reduce the dose of any PIs being taken at the same time.
Sustiva (also known as Stocrin)
Efavirenz
One 600-mg tablet once a day.
Rash; central nervous system symptoms, such as drowsiness, insomnia, confusion, inability to concentrate, dizziness, and vivid dreams; increased liver enzymes; false-positive drug testing (marijuana); and birth defects if taken during pregnancy.
September 17, 1998
Take on an empty stomach at bedtime to reduce side effects. The dosage of some PIs may need to be increased or boosted with Norvir.
Intelence
Etravirine
Two 100-mg tablets twice a day.
Rash, nausea.
June 18, 2008
Take with food. May not be combined with certain Norvir boosted PIs, but other PIs must be boosted with Norvir; Do not use with other NNRTIs.
Viramune
Nevirapine
One 200-mg tablet once a day for 14 days, then one 200-mg tablet twice a day.
Black Box warning: Severe, life-threatening liver problems, notably among women with T-cell counts >250; severe skin reactions; careful dosing and monitoring needed at start of treatment.
June 21, 1996
Take with or without food. Dosage of certain PIs may need to be increased or boosted with Norvir. Do not use with Reyataz.
Atripla
Efavirenz, tenofovir, and emtricitabine
One tablet once a day. Contains two nucleoside reverse transcriptase inhibitors (NRTIs) and one NNRTI in one tablet.
Similar side effects to Sustiva (efavirenz), Viread (tenofovir), and Emtriva (emtricitabine). Please note Viread’s and Emtriva’s Black Box warnings.
August 2, 2004
Can be used with or without other HIV medications. Take on an empty stomach at bedtime to reduce side effects.
PROTEASE INHIBITORS (PIs)
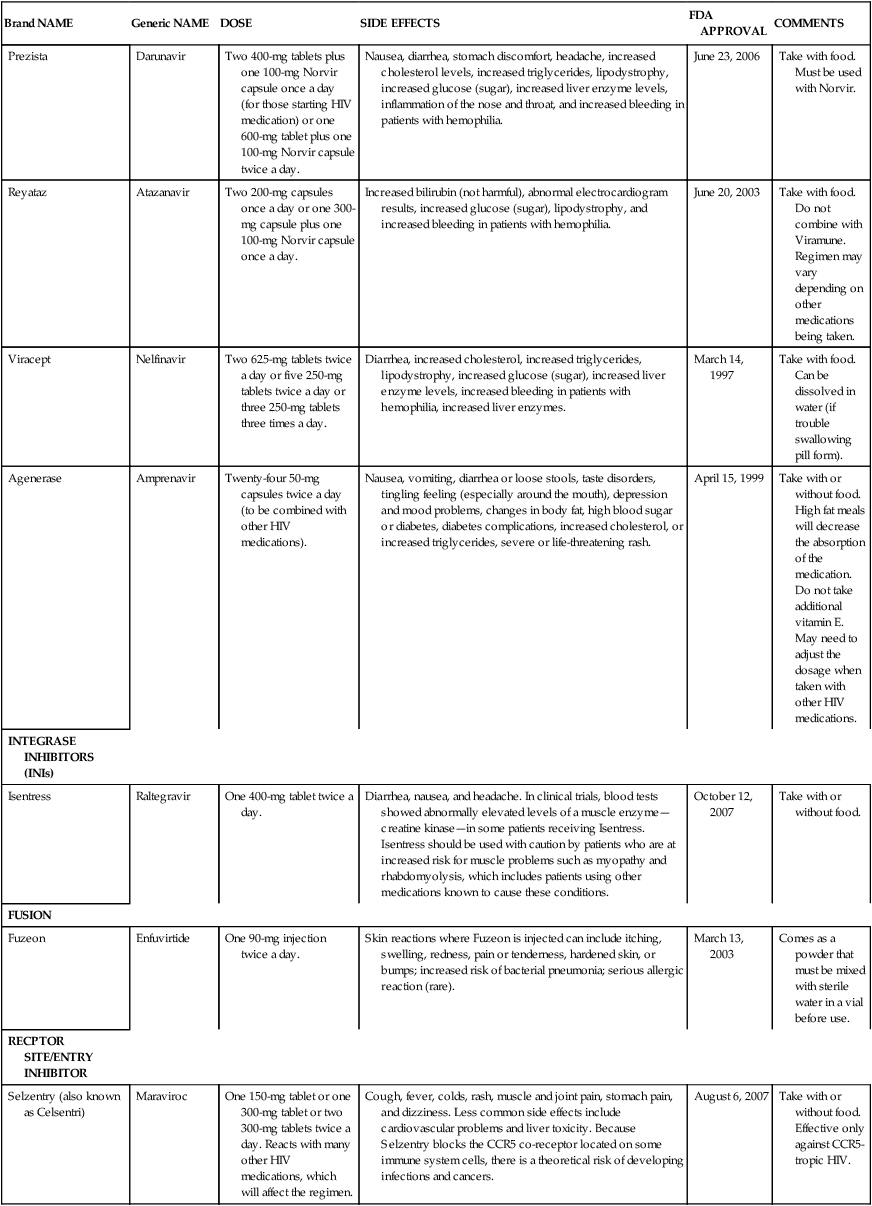
Aptivus
Tipranavir
Two 250-mg capsules plus two 100-mg Norvir capsules twice a day.
June 22, 2005
Take with food. Approved only for treatment-experienced patients. Do not take with other PIs except for Norvir.
Crixivan
Indinavir
Two 400-mg capsules twice a day; preferred regimen is two 400-mg capsules twice a day plus one or two 100-mg Norvir capsules twice a day.
Kidney stones, nausea, vomiting, diarrhea, increased cholesterol, increased triglycerides, increased glucose (sugar), lipodystrophy, increased bilirubin (not harmful), increased bleeding in patients with hemophilia. Others: headache, weakness, blurred vision, dizziness, rash, metallic taste, low platelets, hair loss, anemia.
March 13, 1996
Take on an empty stomach or with a light, low-fat snack. If taking the preferred dose, take with or without food. Drink six glasses of water each day to prevent kidney stones.
Invirase
Saquinavir
Two 500-mg capsules plus one 100-mg Norvir capsule twice a day.
Nausea, diarrhea, stomach discomfort, headache, increased cholesterol, increased triglycerides, lipodystrophy, increased glucose (sugar), increased liver enzyme levels, and increased bleeding in patients with hemophilia.
December 6, 1995
Must be used with Norvir. Take with food.
Kaletra (also known as Aluvia)
Lopinavir and ritonavir
Two tablets twice a day or four tablets once a day. Contains two PIs in one tablet.
Nausea, diarrhea, stomach discomfort, headache, increased cholesterol, increased triglycerides, lipodystrophy, increased glucose (sugar), increased liver enzyme levels, and increased bleeding in patients with hemophilia.
September 15, 2000
Take with or without food. Must be taken twice a day and dose may need to be increased if taken with certain other medications.
Lexiva (also known as Telzir)
Fosamprenavir
Two 700-mg tablets twice a day or two 700-mg tablets plus one 100-mg Norvir capsule once or twice a day.
Skin rash, nausea, diarrhea, stomach discomfort, headache, increased cholesterol, increased triglycerides, lipodystrophy, increased glucose (sugar), increased liver enzyme levels, and increased bleeding in patients with hemophilia.
October 20, 2003
Take with or without food. If other PIs have been taken in the past, only take the twice-a-day, Norvir boosted combination.
Norvir
Ritonavir
Six 100-mg capsules twice a day.
Nausea, vomiting, diarrhea, appetite loss, numbness or tingling around the mouth, increased cholesterol, increased triglycerides, lipodystrophy, and diabetes.
March 1, 1996
The full dose is rarely used. It is most often used to boost the levels of other PIs in the blood. Needs refrigeration in hot weather.
Prezista
Darunavir
Two 400-mg tablets plus one 100-mg Norvir capsule once a day (for those starting HIV medication) or one 600-mg tablet plus one 100-mg Norvir capsule twice a day.
Nausea, diarrhea, stomach discomfort, headache, increased cholesterol levels, increased triglycerides, lipodystrophy, increased glucose (sugar), increased liver enzyme levels, inflammation of the nose and throat, and increased bleeding in patients with hemophilia.
June 23, 2006
Take with food. Must be used with Norvir.
Reyataz
Atazanavir
Two 200-mg capsules once a day or one 300-mg capsule plus one 100-mg Norvir capsule once a day.
Increased bilirubin (not harmful), abnormal electrocardiogram results, increased glucose (sugar), lipodystrophy, and increased bleeding in patients with hemophilia.
June 20, 2003
Take with food. Do not combine with Viramune. Regimen may vary depending on other medications being taken.
Viracept
Nelfinavir
Two 625-mg tablets twice a day or five 250-mg tablets twice a day or three 250-mg tablets three times a day.
Diarrhea, increased cholesterol, increased triglycerides, lipodystrophy, increased glucose (sugar), increased liver enzyme levels, increased bleeding in patients with hemophilia, increased liver enzymes.
March 14, 1997
Take with food. Can be dissolved in water (if trouble swallowing pill form).
Agenerase
Amprenavir
Twenty-four 50-mg capsules twice a day (to be combined with other HIV medications).
Nausea, vomiting, diarrhea or loose stools, taste disorders, tingling feeling (especially around the mouth), depression and mood problems, changes in body fat, high blood sugar or diabetes, diabetes complications, increased cholesterol, or increased triglycerides, severe or life-threatening rash.
April 15, 1999
Take with or without food. High fat meals will decrease the absorption of the medication. Do not take additional vitamin E. May need to adjust the dosage when taken with other HIV medications.
INTEGRASE INHIBITORS (INIs)
Isentress
Raltegravir
One 400-mg tablet twice a day.
Diarrhea, nausea, and headache. In clinical trials, blood tests showed abnormally elevated levels of a muscle enzyme—creatine kinase—in some patients receiving Isentress. Isentress should be used with caution by patients who are at increased risk for muscle problems such as myopathy and rhabdomyolysis, which includes patients using other medications known to cause these conditions.
October 12, 2007
Take with or without food.
FUSION
Fuzeon
Enfuvirtide
One 90-mg injection twice a day.
Skin reactions where Fuzeon is injected can include itching, swelling, redness, pain or tenderness, hardened skin, or bumps; increased risk of bacterial pneumonia; serious allergic reaction (rare).
March 13, 2003
Comes as a powder that must be mixed with sterile water in a vial before use.
RECPTOR SITE/ENTRY INHIBITOR
Selzentry (also known as Celsentri)
Maraviroc
One 150-mg tablet or one 300-mg tablet or two 300-mg tablets twice a day. Reacts with many other HIV medications, which will affect the regimen.
Cough, fever, colds, rash, muscle and joint pain, stomach pain, and dizziness. Less common side effects include cardiovascular problems and liver toxicity. Because Selzentry blocks the CCR5 co-receptor located on some immune system cells, there is a theoretical risk of developing infections and cancers.
August 6, 2007
Take with or without food. Effective only against CCR5-tropic HIV.
Viral load measurement
Vaccines
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Human immunodeficiency virus infection: living with A chronic illness
Only gold members can continue reading. Log In or Register to continue

