CHAPTER 78 Hematologic Arthritis
HEMOPHILIC ARTHROPATHY
The elbow is frequently involved with acute hemarthrosis.14 In addition, the elbow is the second most common site of arthropathy in the hemophilic patient after the knee joint. A 1965 report from Sweden surveyed 114 patients with hemophilia A and 43 patients with hemophilia B.1 Sixty-six of the 95 severely affected and 20 of the 38 moderately affected patients had elbow involvement; 59 patients had bilateral elbow involvement. The severity of the arthropathy in all joints increased with age and disease severity.
PATHOGENESIS
Hemophilia is an X-chromosome–linked disease characterized by a deficiency or functional defect of coagulation factors VIII or IX, which results in an increased bleeding tendency. Hemophilic arthropathy is believed to be the consequence of repeated episodes of intra-articular bleeding, which can occur spontaneously or as a result of trauma.21 Joint destruction is the result of the direct deleterious effect of joint hematoma on the metabolism of the articular cartilage and the secondary hypertrophic synovitis, which leads to cartilage and bone destruction as well as soft tissue contractures (Box 78-1). Increases in intra-articular pressure may also play a role in producing the degenerative changes.39 The growth plates may be affected in children, which may result in associated angular deformity. The pathogenesis and management of hemophilic arthropathy may be further complicated by the presence of a coexistent human immunodeficiency virus (HIV) infection.10
EVALUATION
Patients with hemophilic arthropathy of the elbow may present in various stages of the disease. The first episodes of acute elbow hemarthrosis present with severe pain, effusion, and limited motion. As joint degeneration progresses, patients develop various grades of pain, stiffness, and deformity. Gamble et al18 measured elbow motion in 48 patients with hemophilia followed for an average of 10.8 years. Patients older than 25 years had decreased motion in all planes compared with patients younger than 15 years. Pronation was the first motion to be restricted, and extension was the motion most affected at the end of follow-up. Interestingly, many patients with hemophilic arthropathy are more limited by their lack of pronation and supination than by limited flexion and extension.
Hemophilic arthropathy commonly affects other joints including the knees and ankles. In the upper extremity, the elbow is more commonly affected than the shoulder or the wrist; in a series of 23 moderate and severe hemophiliacs, the elbow joints were the site of recognizable arthropathy in 87% of the cases, whereas the shoulder and wrist were affected in a small proportion of patients.21 When surgery is contemplated, attention should be paid to the order in which the different joints need to be addressed.
Hemophilic pseudotumors are an uncommon but well-known musculoskeletal complication of hemophilia.29 Pseudotumors occur in approximately 1% to 2% of patients with bleeding disorders. They present usually as an enlarging mass secondary to intraosseous, subperiosteal or intramuscular bleeding with various degrees of bone and soft tissue compromise. They can compress adjacent neurovascular structures and be misdiagnosed as true malignancies based on their clinical and radiographic features.
Imaging Studies
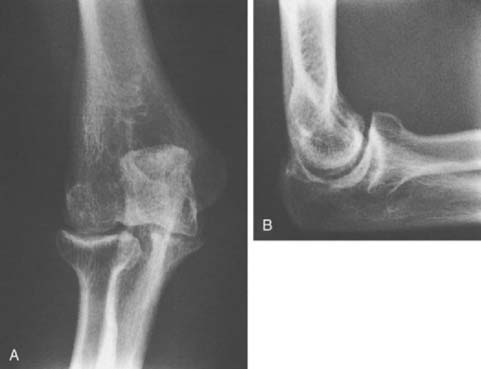
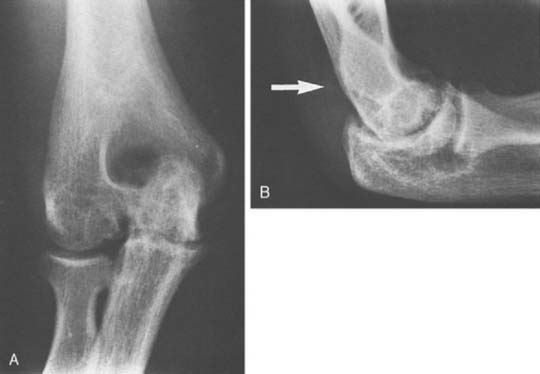
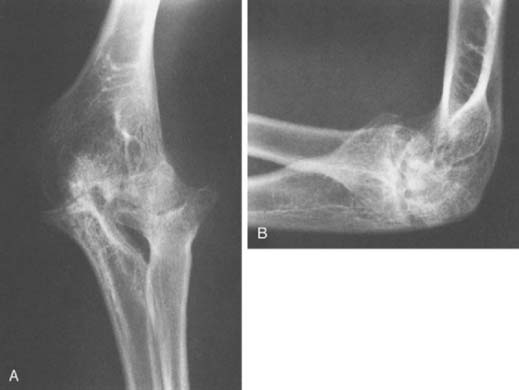
Hemophilic arthropathy usually presents with joint line narrowing, spurs, subperiosteal new bone formation, and occasionally, bone loss or deformity. A number of classification systems have been developed to rate the severity of the radiographic changes in hemophilic arthropathy. The system published by Arnold and Hilgarter3 has been extensively used in the literature and includes five stages as detailed in Box 78-2 (see Figs. 78-1 through 78-4).
BOX 78-2 Radiographic Classification of Hemophilic Arthropathy3
| Stage | Features |
|---|---|
| I | No skeletal abnormalities |
| Soft issue swelling present | |
| II | Osteoporosis, epiphyseal overgrowth |
| Normal joint line | |
| No bone cysts | |
| III | Subchondral joint changes |
| Subchondral cysts visible | |
| Trochlear notch widened | |
| IV | Joint space narrowing |
| V | Loss of joint space |
| Joint contracture | |
| Enlargement of epiphyses or architectural changes |
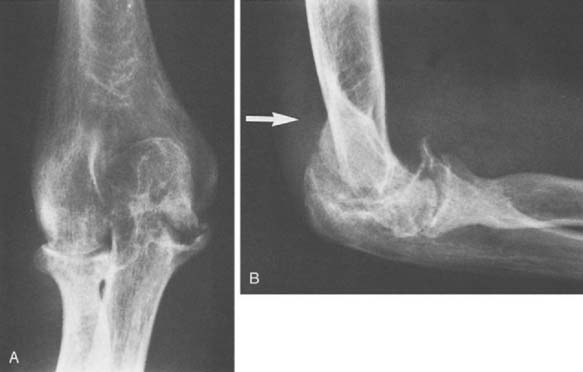
FIGURE 78-3 A, Radiographs of the same patient shown in Figure 78-2 taken 5 years later show progressive changes with loss of the joint space narrowing and loss of cartilage (stage IV). B, Again the arrow shows opacification of the synovium.
More recently, the World Federation of Hemophilia has adopted the radiographic scoring and classification system proposed by Peterson et al32 and based on thefollowing eight aspects: osteoporosis, hypertrophy of the endplate, loss of joint space, irregular subchondral surface, subchondral cysts, erosion of the joint surface, incongruity of the joint surfaces, and deformity of the joint. The score ranges from 0 (no radiographic abnormalities) to 13 (severe arthropathy).
Some authors have identified three main patterns of joint involvement.19,43 Patients with involvement of predominantly the medial side of the joint present with ulnohumeral joint narrowing and medial spurring, which may irritate the ulnar nerve. Involvement of the lateral side of the joint is associated with radial head enlargement, posterolateral elbow pain, and restriction of pronation and supination. Finally, global involvement of the joint is associated usually with more severe stiffness affecting all planes of motion.
As with other inflammatory conditions, magnetic resonance imaging has been used to more precisely evaluate abnormalities of the articular cartilage, soft tissues, and synovium and to monitor the progression of the arthropathy.46 Several scoring systems based on magnetic resonance findings have been developed to identify patients at risk for the development of severe arthropathy and to prevent articular changes by the use of more intensive medical treatment.25,26
TREATMENT
Medical Management
The treatment of hemophilia requires correction of the coagulation defect by replacement of the deficient factor. Patients with mild hemophilia A or Von Willebrand’s disease may benefit from treatment with desmopressin, which releases factor VIII from endothelial storage sites. At present, recombinant concentrates of factors VIII and IX are the products of choice for factor replacement. The regular administration of replacement therapy with the object of reducing spontaneous bleeding is a logical approach. The goal is to maintain the factor VIII or IX level above 1%. This can be achieved by factor concentrate administration two or three times a week. Some physicians recommend factor replacement for all patients, whereas others favor treating only those patients with frequent bleeding episodes. Starting treatment at a younger age seems to protect against later development of hemophilic arthropathy.17
Replacement therapy is also the treatment of choice of bleeding episodes. The desired factor level is a function of the severity and location of the bleeding episode. Joint or muscle bleeding episodes are treated to achieve factor levels of 30% to 50%. Replacement therapy is also critical when surgery is considered.10 Most orthopedic surgery can be managed with initial levels of 80% to 100%, followed in a few days by minimum levels of at least 30%. Joint manipulation under anesthesia requires levels of at least 50%. The number of units required to obtain these titers is calculated based on the body weight and the volume of distribution, which is 1.5 for factor VIII and 1.2 for factor IX. Factor levels should be checked to ensure the effectiveness of the calculated dose. Patients with factor VIII inhibitor require a specific approach; if the inhibitor titer is below 5 BU, recombinant VII factor is administered. If the inhibitor titer is greater than 5 BU, there are three treatment options: porcine factor VIII, factor VIII inhibitor bypassing activity (FEIBA), or recombinant factor VIIa.
Acute Hemarthrosis
The treatment of acute hemarthrosis is mostly medical, and includes factor replacement and analgesia. The goal of replacement therapy is to maintain levels of 50% to 100% (or increase the plasma level between 20% and 50%) until the bleeding stops. Prompt replacement therapy is advised at the earliest sign of bleeding, especially in younger patients, in order to prevent joint degeneration. Well-trained patients and families are able to administer their replacement therapy at home without examination by a physician, which might delay treatment.
Tense painful hemarthrosis may be aspirated under factor replacement coverage. The needle is introduced into the joint usually in the soft spot centered between the radial head, the tip of the olecranon, and the lateral epicondyle. Aspiration should not be performed until the clotting factor has been raised to at least 50%. Uncontrolled massive joint bleeding may be controlled through selective angiographic embolization of the affected blood vessels.30 Acute ulnar nerve compression requiring surgical intervention has been reported as a complication of acute elbow hemarthrosis.27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree