Chapter Six Haemodynamics
Introduction
1. Spinal movements can affect blood flow
2. Disease or injury to blood vessels can present with local pain patterns similar to commonly seen NMS conditions.
• How does movement actually affect blood flow, and in what way (i.e. detrimentally or positively)?
• How can pain arising from a vascular source be differentiated from NMS pain?
Haemodynamics and the cervical spine
Clinical anatomy
The posterior vascular system of the head and neck
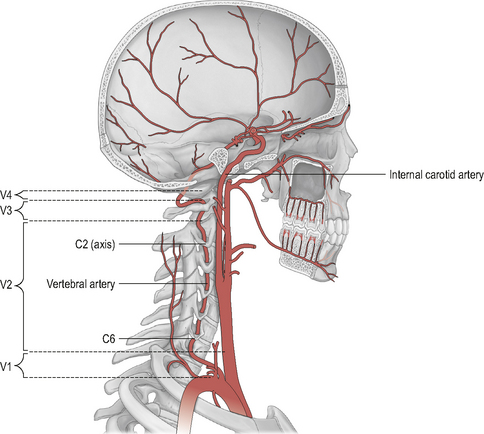
The hind-brain is supplied primarily by the vertebrobasilar complex. Figure 6.1 shows schematically the vertebral and basilar arteries, and how they feed into the Circle of Willis.
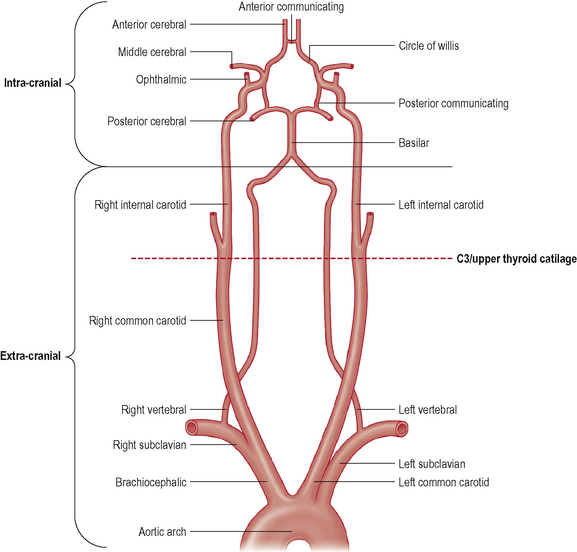
Figure 6.1 • Schematic representation of the vertebral and carotid arteries and their relation with Circle of Willis.
(Adapted and reprinted from Drake et al (2005), with permission from Elsevier)
The verterbral artery (VA) can be divided into extra-cranial and intra-cranial sections (Fig. 6.1). Furthermore, the course of the VA is sub-divided into four anatomical divisions (Fig. 6.2): V1 (extra-cranial) – from the branch origin off the sub-clavian artery to the transverse process of C6, where the artery enters the cervical column; V2 (extra-cranial) – its course through the bony vertebral column (as it passes through the transverse foramina) from C6 to its exit at the atlantal transverse foramen; V3 (extra-cranial) – from the atlantal transverse foramen to its cranial entrance through the atlanto-occipital membrane; and V4 (intra-cranial) – from the foramen magnum to converge with the contralateral VA at the lower pontine hind-brain level to form the basilar artery. The most common sites of VA injury are V1 at its origin, and V3 as it convolutes around the posterior arch of the atlas (Savitz & Caplan, 2005).
The basilar artery (BA) runs through the cisterna pontis in a shallow median groove (known as the sulcus basilaris) from its origin to the superior pontine line (upper pontine level) where it begins to form the Circle of Willis via its bifurcation into the two posterior cerebral arteries. For a more detailed description of the vertebrobasilar arterial system, together with the many anatomical anomalies which have been noted, see Rivett (2005).
Studies examining changes of blood flow in the posterior system produce variable results. Many studies have demonstrated a change in blood flow in the vertebral arteries during contralateral cervical rotation (e.g. Mitchell et al, 2004; Refshauge, 1994; Rivett, 1999; Rossitti & Volmann, 1995). Although there are other studies with contradictory results, contralateral rotation is the movement most consistently associated with reduction (or cessation) of VA blood flow. It is important to realize, however, that this phenomenon is likely to be a physiologically natural event and occurs in normal, asymptomatic individuals.
The anterior vascular system of the head and neck
Figure 6.1 also shows the course of the internal carotid artery (ICA) from its origin (around the C3/upper thyroid cartilage level) to its termination at the Circle of Willis. The ICA arises from the common carotid artery (CCA) which itself is a branch off the thoracic arch of the aorta (on the left), or the brachiocephalic artery (on the right). Like the VAs, the ICA can be divided into extra- and intra-cranial sections. Extra-cranially, the ICA begins at its bifurcation. The point of bifurcation is of great clinical importance for a number of reasons. The carotid sinus is located just superior to the bifurcation on the ICA. The carotid sinus houses nerve endings from the glossopharangeal nerve (IX) and acts as a baroreceptor controlling intra-cranial blood pressure. This is also a very common site for localized atherosclerotic lesions (Lorenz et al, 2006). The carotid sinus is behind the point of bifurcation and this acts as a chemoreceptor. The bifurcation area sits deep to a number of muscles which are active during cervical spine movement, jaw movement, and swallowing. This is known as the carotid triangle (a sub-division of the anterior cervical triangle) and it is where the vessels are at their most superficial placement. The carotid triangle consists of the superior belly of omohyoid (antero-inferior border), stylohyoid and digastric (superior border), and the anterior aspect of sternocleidomastoid (posterior border). Flow changes around this section of the ICA have been demonstrated during movements that involve activity and stretching of these muscles (e.g. Foye et al, 2002).
Although blood flow in the anterior vessels is commonly measured and reported upon, it is mostly in relation to the effect of disease (i.e. stenotic lesions reduce blood flow). Studies specifically examining the effect of cervical movement on carotid blood flow are less common than those examining posterior flow. However, there are several studies which demonstrate that carotid flow can be influenced (reduced) by cervical extension, and to a smaller extent, rotation (e.g. Rivett, 1999; Scheel et al, 2000; Schoning et al, 1994).
Cranial nerves
It is important to appreciate the relationship between cervico-cranial blood flow and the cranial nerves (CN). Just as with peripheral nerves, these structures require disproportionally high amounts of blood and constant perfusion. Any interruption to this perfusion will result in symptoms of neural ischaemia. It is therefore essential that the clinician understands the roles of each cranial nerve and the manifestations of dysfunction to each nerve, as well as the specific cranial nerve tests. As the nuclei to most cranial nerves are housed in the brainstem, disruption to vertebrobasilar flow can potentially result in cranial nerve dysfunction of these nerves. ICA flow changes are unlikely to affect the nuclei of the cranial nerves, but, by virtue of the relative anatomy, can affect the nerve axons in both the extra- and intra-cranial sections of the ICA following certain vessel pathologies. The last four cranial nerves (CNIX to XII) have been reported as affected in 83% of ICA traumatic events (Chan et al, 2001).
Pathophysiology
Ischaemic strokes (as opposed to hemorrhagic strokes) account for around 80% of all young to middle-aged strokes. The majority of these strokes arise from the internal carotid artery whilst around 20% arise from the posterior system (Arnold & Bousser, 2005; Savitz & Caplan, 2005; Thanvi et al, 2005). These figures relate specifically to dissection events. Dissections are intimal tears that allow blood to penetrate into the vessel wall (Fig. 6.3).
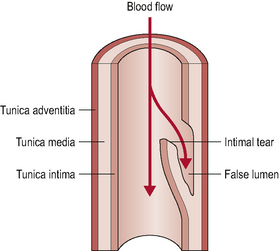
Figure 6.3 • Arterial dissection. The intimal tear allows blood to track sub-intimally and create a false lumen.
(Adapted with permission from McKeeson Health Solutions 2002)
These dissections may be sub-intimal, which may result in intramural haematoma formation and subsequent lumen narrowing as the intima wall is enlarged into the lumen. Others may be sub-adventitial, which may result in a gross widening of the vessel referred to as a dissecting aneurysm. There is potential for the resultant thrombus (haematoma) formation to either enlarge to the point of clinically significant stenosis or to embolize (also referred to as dissecting of the thrombus). A widened vessel, and the associated inflammation in its proximity, can also compress or stretch local structures resulting in a variety of symptoms, including somatic pain from non-vascular structures (Arnold & Bousser, 2005) or cranial nerve dysfunction/palsy (Leys et al, 1997). Vasculogenic pain may also arise from the deformation of nociceptive nerve endings in the adventitia of the vessel, as a result of vessel widening (Nichols et al, 1993).
Atherosclerosis is intrinsically associated with intimal dysfunction and the presence of atherosclerosis may predispose the vessel to the above pathological reactions and make dissection and thrombus formation more likely to occur (Mitchell, 2002). Furthermore, localized atherosclerotic changes may occur as a result of the extrinsic or intrinsic (altered haemodynamic) trauma which is responsible for the above intimal tears (Texon, 1996).
Clinical presentations
Posterior circulation presentations
These involve the posterior vertebrobasilar system as described above. Classically, the signs and symptoms related to the posterior system are considered as the ‘5 Ds and 3 Ns’ of Coman (Coman, 1986). The signs and symptoms are presented in Table 6.1 (with a ninth ‘classic’ sign – ataxia) together with the associated neuro-anatomical site of insult.
Table 6.1 Classic signs and symptoms of vertebrobasilar insufficiency (VBI) with associated neuroanatomy. See text for the limitations of only considering these features for potential VBI
| Sign or symptom | Associated neuroanatomy |
|---|---|
| Dizziness (disequilibrium, giddiness, lightheadedness) | Lower vestibular nuclei (vestibular ganglion = nuclei of CN VIII vestibular branch) |
| Drop attacks (loss of consciousness) | |
| Diplopia (amaurosis fugax; corneal reflux) | Descending spinal tract, descending sympathetic tracts (Horner’s syndrome); CN V nucleus (trigeminal ganglion) |
| Dysarthria (speech difficulties) | CN XII nucleus (medulla, trigeminal ganglion) |
| Dysphagia (+ hoarseness/hiccups) | Nucleus ambiguous of CN IX and X, medulla |
| Ataxia | Inferior cerebellar peduncle |
| Nausea | Lower vestibular nuclei |
| Numbness (unilateral) | |
| Nystagmus | Lower vestibular nuclei + various other sites depending on type of nystagmus (at least 20 types) |
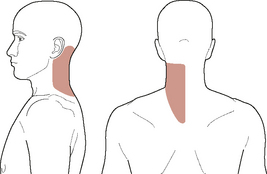
Unreasoned adherence to these cardinal ‘classic’ signs and symptoms can, however, be misleading and result in an incomplete understanding of patient presentations. A closer look at contemporary evidence and case reports shows that the typical presentation of vertebrobasilar dysfunction is not always in line with this classic picture. Clinical haemodynamic presentations can be better understood if the symptomology is broken down into non-ischaemic (i.e. local, somatic causes) and ischaemic (i.e. brain, or retinal) manifestations (Arnold & Bousser, 2005). The non-ischaemic presentation of vertebral dissection is typically ipsilateral posterior neck pain and/or occipital headache alone (e.g. Arnold & Bousser, 2005; Asavasopon et al, 2005; Childs et al, 2005; Dziewas et al, 2006; Leys et al, 1997; Nichols et al, 1993; Savitz & Caplan, 2005; Silbert et al, 1995; Thanvi et al, 2005; Watanabe et al, 2001). Figure 6.4 shows a typical pain distribution for vertebral artery dissection.
This stage may then be followed by the ischaemic events associated with vertebrobasilar dysfunction. These may also include some of the classic 5Ds and 3Ns as stated above, but may also include any of the following (APA, 2006; Arnold & Bousser, 2005; Rivett, 2005; Savitz & Caplan, 2005):
• Hypotonia/limb weakness (arm or leg)
• Anhidrosis (lack of facial sweating)
• Clumsiness and agitation, anxiety
It is rare for posterior dysfunction to manifest in only one sign or symptom, and isolated dizziness or transient loss of consciousness are often misattributed to posterior circulation ischaemia (Savitz & Caplan, 2005). The nature of dizziness is a differentiating factor in establishing a vascular versus non-vascular cause. Typically, posterior circulation dizziness (as a result of the subsequent occluded flow through the AICA and labyrinthine arteries), does not present as frank vertigo (although some authors have suggested this could occur, e.g. Savitz and Caplan (2005). Rotation of the torso and neck as a single unit (i.e. no cervical torsion) may assist in differentiating vascular from vestibular causes of dizziness. Further detail regarding vestibular testing is beyond the scope of this chapter.
Anterior circulation presentations
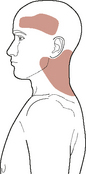
Fronto-temporal headache (cluster-like, thunder-clap, migraine without aura, hemicrania continua, different from previous headaches), upper cervical or anterolateral neck pain, facial pain/facial sensitivity (‘carotidynia’), Horner’s syndrome, pulsatile tinnitus, and cranial nerve palsies (most commonly CN IX to XIII) are the commonest local sign/symptoms presentations of internal carotid dissection/thrombus formation (Arnold & Bousser, 2005; Taylor & Kerry, 2005; Zetterling et al, 2000). Figure 6.5 shows a typical ICA-referred pain distribution.
Less common local signs and symptoms include ipsilateral carotid bruit, scalp tenderness, neck swelling, CN VI palsy, orbital pain, and anhidrosis (Frigerio et al, 2003; Guillon et al, 1998; Lemesle et al, 1998; Zetterling et al, 2000). It is important to appreciate that most commonly, particularly in the early stages of the pathology, headache and/or cervical pain can be the sole presentations of internal carotid artery dysfunction (Biousse et al, 1994; Buyle et al, 2001; Lemesle et al, 1998; Mainardi et al, 2002; Nichols et al, 1993; Pezzini et al, 2005; Rogalewski & Evers, 2005; Silbert et al, 1995; Taylor & Kerry, 2005).
The local pain mechanisms involved with the internal carotid artery are again likely to be related to either deformation of nerve endings in the tunica-adventitia, or direct compression on local somatic structures (Nichols et al, 1993). Specifically, the terminal nerve endings in the carotid wall are supplied by the trigeminal nerve, which accounts for instances of facial pain and carotidynia. Stimulation of the trigemino-vascular system may account for this carotid-induced pain (Leira et al, 2001).
Cranial nerve palsies and Horner’s syndrome are interesting phenomena and often pathognomonic of internal carotid artery pathology, especially if the onset is acute. The hypoglossal nerve (CN XII) is the most commonly affected, followed by the glossopharyngeal (CN IX), vagus (CN X), or accessory (CN XI) (Arnold & Bousser, 2005; Zetterling et al, 2000). However, all cranial nerves (except the olfactory nerve) can be affected (Zetterling et al, 2000). If the dissection extends into the cavernous sinus, the occulomotor (CN III), trochlear (CN IV), or abducens (CN VI) can be affected (Lemesle et al, 1998; Zetterling et al, 2000). The two most likely mechanisms for these cranial nerve palsies are (1) ischaemia to the nerve via the vasa nervorum (comparable to peripheral neurodynamic theory); and (2) direct compression of the nerve axon by the enlarged vessel (Arnold & Bousser, 2005; Lemesle et al, 1998; Zetterling et al, 2000).
Horner’s syndrome has been found to be present in up to 82% of patients with known internal carotid dissection (Chan et al, 2001). Most commonly, this syndrome occurs with head, neck, or facial pain. Carotid-induced Horner’s syndrome manifests as a drooping eyelid (ptosis), sunken eye (enophthalmia), and a small, constricted pupil (miosis) and facial dryness (anhidrosis), i.e. the overbalance of parasympathetic activity in the eye. The syndrome is therefore a result of interruption to the sympathetic nerve fibres supplying the eye. In the case of carotid Horner’s syndrome, the pathology is classed as post-ganglionic. The superior cervical sympathetic ganglion lies in the posterior wall of the carotid sheath, and the post-ganglionic fibres follow the course of the carotid artery before making their way deep towards the eye through the cavernous sinus. Compression or ischaemia as a result of internal carotid dysfunction will occur at the ganglion or distal to it. Some post-ganglionic sympathetic fibres that follow the course of the external carotid artery control facial sweating, thus accounting for the presence of anhidrosis in post-ganglionic Horner’s syndrome.
The above local signs and symptoms of internal carotid pathology can precede cerebral ischaemia (TIA or stroke) or retinal ischaemia by anything from less than a week, to beyond 30 days (Biousse et al, 1994; Zetterling et al, 2000). In addition to the above early signs, it is important for the manual therapist to be aware of signs and symptoms related to cerebral and retinal ischaemia (i.e. internal carotid territory). It is unlikely that a patient with full stage cerebral ischaemic stroke will present to the manual therapist, but the more subtle presentation of retinal ischaemia might. The internal carotid artery supplies (via the ophthalmic artery) the retina, and embolus from the internal carotid can result in retinal ischaemic dysfunction: symptoms include a painless episodic loss of vision, or blackout (amaurosis fugax), and localized/patchy blurring of vision (scintillating scotoma). Orbital ischaemia syndrome, as a result of ophthalmic artery occlusion, presents as weakness of the ocular muscles (ophthalmoparesis); protrusion of the eye due to weakness of extrinsic eye muscles (proptosis); swelling of the eye or conjunctiva (chemosis) (Arnold & Bousser, 2005; Dziewas et al, 2006; Zetterling et al, 2000).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree