A Andry’s Tree
A knowledge of normal and abnormal growth and development is vital to an understanding of pediatric orthopedics [B]. This knowledge increases our comprehension of the musculoskeletal system, improves our understanding of the causes of disease, and makes us better able to manage the varied orthopedic problems of childhood.

B Femoral torsion Femoral torsion is often familial. Many common musculoskeletal problems have a genetic basis.
Dividing the period of growth into six stages provides a convenient framework to review both normal and abnormal growth and development [C]. During the first stage, reproductive cells or gametes are formed.

C Growth phase The period of growth can be divided into six phases.
Normal Growth
During normal development, cells proliferate, undergo differentiation, move, and even in some cases die in order to produce a normal, mature individual.
Gamete
Gamete is a collective term for ovum and sperm. During gametogenesis, meiotic division halves the chromosome number. Genetic material, which may include defective genes, is shuffled, and mature ova and sperm are formed [D].
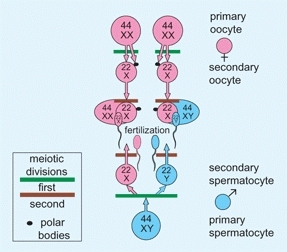
D Gametogenesis The ovum and sperm are formed by two meiotic divisions that halve the chromosome number and shuffle genetic material. Fertilization combines the traits of both parents to create a unique individual.
Early Embryo
This early embryonic phase encompasses the 2-week period from fertilization to the implantation of the embryo.
First week
During the first week following fertilization, the zygote repeatedly divides as it moves through the fallopian tubes to the uterus. The zygote becomes a morula, then a blastocyst. The blastocyst implants itself on the posterior uterine wall.
Second week
During this week, the amniotic cavity and trilaminar embryonic disc are formed [A]. The early embryo is usually aborted if a lethal or serious genetic defect is present. During these first two weeks, the early embryo is less susceptible to teratogens than during the following embryonic period.
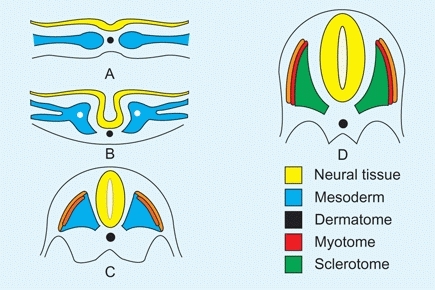
A Trilaminar disc The neural tube closes. The mesoderm differentiates into dermatome, myotome, and sclerotome.
Embryo
The embryonic period is characterized by rapid cell activity and organ formation. Cells differentiate and mature, often through induction, a process by which surrounding cells act on other cells to produce entirely new cells or tissue.
Third week
This is the first week of organogenesis. During this week, the trilaminar embryonic disc develops, somites begin to form, and the neural plate closes to form a neural tube.
Fourth week
During this week, the limb buds become recognizable [B]. Somites differentiate into three segments. The dermatome becomes skin, the myotome becomes muscle, and the sclerotome becomes cartilage and bone. The apical ectodermal ridge develops in the distal end of each limb bud. The ridge has an inductive influence on limb mesenchyme, which promotes growth and development of the limb. Serious defects in limb development may originate at this time.
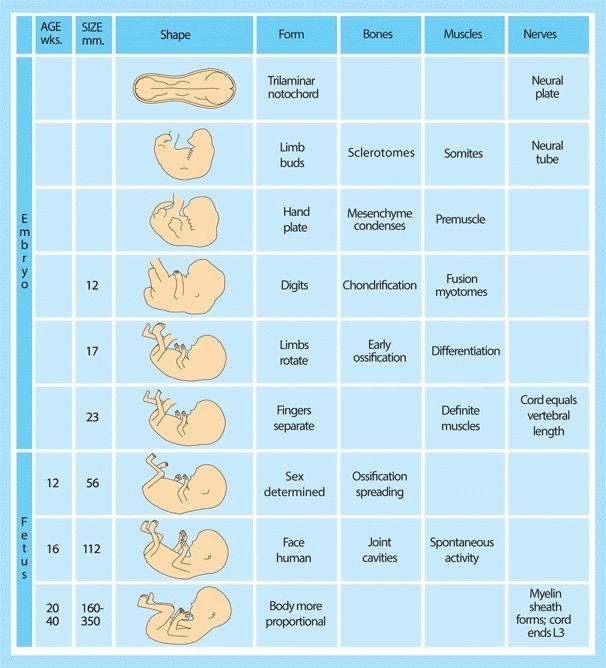
B Prenatal development This chart summarizes musculoskeletal development during embryonic and fetal life.
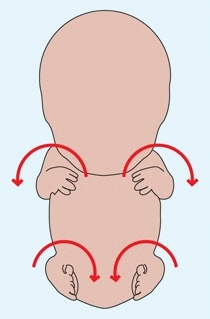
C Limb rotation During the seventh week, the upper limb rotates laterally. The lower limb rotates medially to bring the great toes to the midline.
Fifth week
The hand plate forms and mesenchymal condensations occur in the limbs.
Sixth week
The rays of the digits become evident and chondrification of mesenchymal condensations occurs.
Seventh week
The notches appear between the digit rays. This process, responsible for joint formation, results from cell death. Failure of the separation of rays results in syndactylism. During this week, the upper and lower limbs rotate in opposite directions [C, previous page]. The lower limb rotates medially to bring the great toes to the midline, whereas the upper limb rotates about 90° laterally to position the thumb on the lateral side of the limb.
Eighth week
The fingers separate completely, the embryo assumes a human appearance, and the basic organ systems are completed.
Fetus
The fetal period is characterized by rapid growth and changes in body proportions.
Ninth to twelfth weeks
The first bone, the clavicle, ossifies by a process of intramembranous deposition of calcium. The skeleton develops in a cranial to caudad sequence, with the upper extremities developing before the lower limbs. This results in the upper limbs becoming proportionate compared to the rest of the body, but the lower limbs remaining short.
Thirteenth to twentieth weeks
Growth continues to be rapid. The lower limbs become proportionate, and most bones ossify. The fetal period is characterized by rapid growth and changes in body proportions
Twentieth to fortieth weeks
Growth continues and body proportions become more infant-like.
Connective Tissue
During early fetal life, the basic structure of connective tissue is formed largely of two families of macromolecules: collagens and proteoglycans.
Collagen
Collagen is a family of proteins containing a triple helix of peptide chains [A]. Although at least fifteen different types of collagen are known, five types are most common [B].
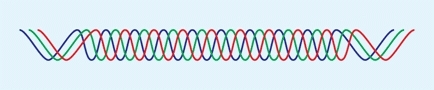
A Collagen helix A triple helix of peptide chains forms the basic collagen structure.
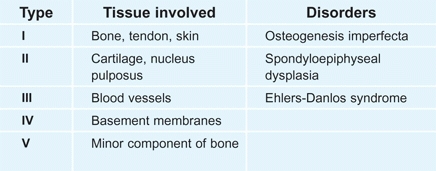
B Collagen types Of the numerous collagen types, five are most widely distributed.
The biosynthesis of collagen
starts in the endoplasmic reticulum, where the basic molecule is assembled. In the extracellular space, procollagen is formed. It is arranged into fibrils and reinforced by cross-linkages to become collagen. Collagen is the major component of connective tissue.
Disorders of collagen are common
They may be minor, producing only increased joint laxity [C], or severe, causing considerable disability. The major collagen disorders are classified according to the site of the defect in the pathway of collagen biosynthesis.
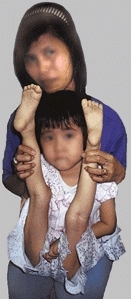
C Clinical manifestations of collagen types Variations of collagen types are common in pediatric orthopedics. This child has developmental hip dysplasia with extreme joint laxity.
Proteoglycans (mucopolysaccharides)
Proteoglycans are macromolecules that form the intracellular matrix of hyaline cartilage and the other connective tissues. Polypeptides or proteins attach to glycosaminoglycan to become proteoglycans [D]. Proteoglycans attach to a hyaluronic acid by a link protein to become an aggregate with a molecular weight in excess of one million. Proteoglycans are highly hydrophilic, and in water they combine with many times their weight of water to create an elastic matrix that is ideal for joint lining. Hyaline cartilage is composed of about equal amounts of proteoglycans and collagen, and it combines with about three times its weight of water, providing a resilient tissue with excellent shock absorbing characteristics. Defects in the formation of these complex molecules produce a variety of diseases.
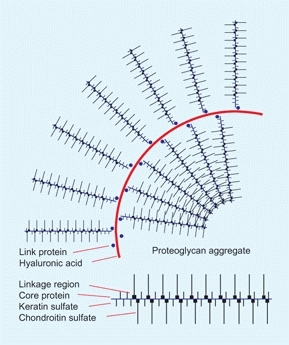
D Proteoglycan aggregate These massive molecules combine with water to form a resilient matrix such as that of hyaline cartilage.
Mucopolysaccharide (MPS) storage diseases result from a deficiency of specific lysosomal enzymes necessary for the degradation of glycosaminoglycans. These diseases are caused by excessive intracellular accumulation of partially degraded molecules that result in conditions such as avascular necrosis or spinal cord compression.
Joints
Synovial joints develop first as a cleft in the mesenchyme, which then chondrifies and cavitates [A]. Cavitation is completed by about the fourteenth gestational week, with the inner mesenchyme becoming synovium and the outer mesenchyme becoming the joint capsule. Normal joint development requires motion, and motion requires a functioning neuromuscular system. Thus, defective joints are often seen in infants with neuromuscular disorders such as myelodysplasia or amyoplasia.
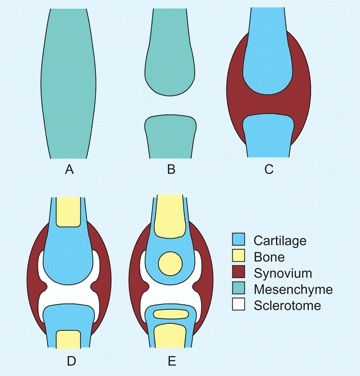
A Synovial joint formation The synovial joints form first as condensations of mesenchyme. Cavitation, chondrification, synovial differentiation, and finally ossification complete the basic structure.
Bone
Bones are formed by osteogenesis. The mandible and clavicle are first formed starting in the seventh gestational week by intramembranous ossification.
Intramembranous Ossification
Osteoblasts differentiate from mesenchyme to form bone directly without a cartilaginous stage. Further growth occurs by appositional bone formation.
Endochondral Ossification
During the sixth gestational week, mesenchymal cells differentiate, condense, and transform into chondrocytes to form a model of the future skeleton. In the center of this model, chondrocytes hypertrophy and begin to calcify. During the next week, a periosteal sleeve of bone is formed, and by the eighth week, vascularization is under way [B].
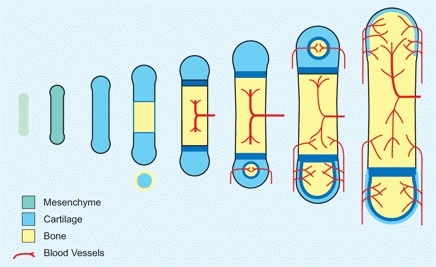
B Endochondral ossification A typical long bone is preformed in mesenchyme. Chondrification precedes ossification.
During the fetal period, primary ossification centers develop in long bones within the diaphysis [C]. Ossification first occurs under the perichondrium. Within the cartilage, hypertrophied cells degenerate. Next, vascular ingrowth occurs, and then the core of the cartilage model is ossified to form the primary ossification center. Endochondral ossification proceeds at the cartilage–bone interphase. Later, secondary ossification centers develop at the ends of the bones, and the cartilage interposed between the primary and secondary ossification centers becomes the growth plate.
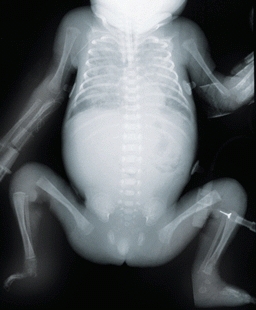
C Radiograph of bones of a newborn infant This radiograph shows primary ossification of the skeleton. Much of the skeleton is cartilage at this age.
Except for the clavicle, all bones of the axial and appendicular skeleton are preformed in cartilage and converted to bone by enchondral ossification. This process begins in the scapula, humerus, radius, and ulna. Ossification continues in an orderly fashion, with centers appearing at different ages. Ossification is earlier in girls than boys [D].
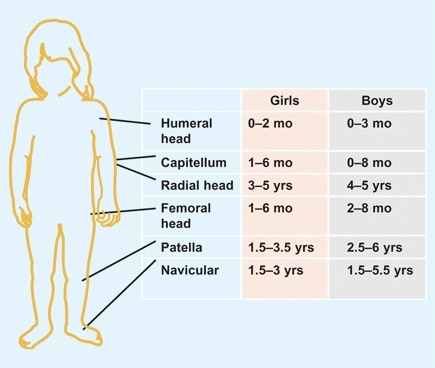
D Ossification Appearance of important ossification centers in girls and boys.
Periosteum
The periosteum seems to develop from the perichondrium. The periosteum is osteogenic, allowing appositional bone growth, and provides a thick sleeve adding resilience and strength to the growing skeleton. This osteogenic capability results in rapid fracture healing and bone regeneration.
Bone Types
Growing bone possesses special characteristics, providing the infant and child with greater flexibility and resilience than that of the adult.
Woven bone
is formed during the fetal period. This bone has less structure, a relatively higher collagen content, and more flexibility than lamellar bone. Woven bone makes up the metaphysis of growing bones, and the type of bone making up the callus following fracture. This flexibility becomes essential during the traverse of the birth canal.
Lamellar bone
Once the need for this high degree of flexibility has passed, woven bone is gradually replaced by lamellar bone starting soon after birth. By four years of age, most woven bone has been converted to lamellar bone.
Ossification Centers
Primary ossification centers
for long bones usually develop before birth [C, previous page], whereas primary ossification centers for smaller bones, such as the patella and most carpal and tarsal bones, develop during infancy [A].
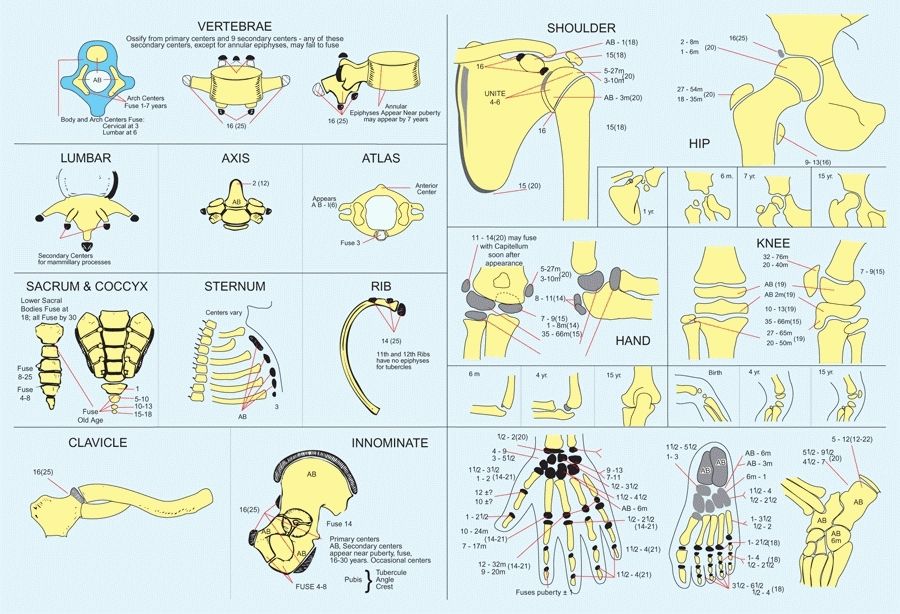
A Ossification centers Redrawn from Girdany BR and Golden R AJR 68:922, 1952.
Secondary ossification centers
develop during infancy and early childhood. They fuse with the primary centers during late childhood, adolescence, and early adult life. Because osseous maturation continues throughout childhood and adolescence in a reasonably orderly fashion, the extent of ossification, as radiographically documented, has become the standard for assessing maturation.
Bone Growth
Cortical thickness increases throughout childhood. For example, the diameter of the diaphysis of the femur increases faster than does the diameter of the medullary canal. This produces an increasing diaphyseal thickness with advancing age. This increasing thickness, lamellar structure, and proportion of calcium give mature bone great tensile strength but little flexibility. These changes are important factors in producing the varying patterns of skeletal injury seen during infancy, childhood, and adult life.
Growth Plate
The growth plate of long bones develops between the primary and secondary ossification centers [A]. The growth plate is responsible for the longitudinal growth of long bones. Growth plate chondrocytes go through an orderly process of proliferation and differentiation followed by terminal differentiation and the formation of new bone. Growth plates with more limited growth potential develop at other sites. These include the periphery of round bones, such as the tarsal bones or vertebral bodies, and the sites of muscle attachments, such as the margins of the ilium. Such sites are referred to as apophyses.
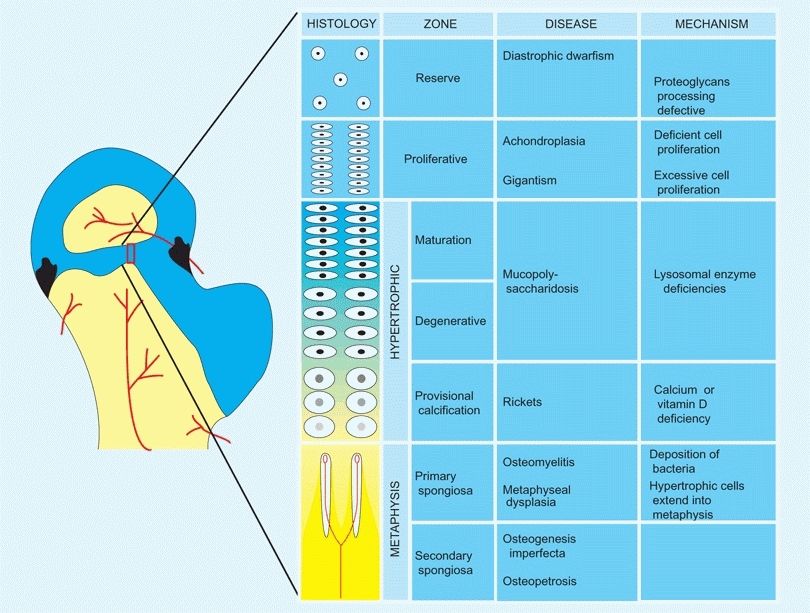
A Growth plate This section from the proximal femoral epiphysis is enlarged to show the histology and disordered growth that occurs at various levels of the growth plate.
The typical long bone epiphysis is divided into zones that reflect morphological, metabolic, and functional differences.
The Reserve Zone (RZ)
This zone is adjacent to the secondary ossification centers and is a zone of relative inactivity. The RZ does not participate in the longitudinal growth of the bone, but it does provide some matrix production and storage functions. This zone contains proteins and genes important in specifying the chondrocyte phenotype, such as SOX–9.
The Proliferative Zone (PZ)
This is the zone of cartilage cell replication and growth. A high metabolic rate and abundant blood supply, oxygen, glycogen, ATP, and collagen make this rapid growth possible. Signaling pathways, important in regulating cell proliferation, are found in this zone. These include insulin-like growth factors and fibroblastic growth factors; both factors are active in this zone.
The Hypertrophic Zone (HZ)
This zone consists of three sub-zones: maturation, degeneration, and provisional calcification segments. In the HZ, the cartilage cells increase in size and the matrix is prepared for calcification. This is associated with a decline in blood supply, oxygenation, and glycogen stores, and with a disintegration of aggregated mucopolysaccharides and chondrocytes. In the sub-zone of provisional calcification, a unique collagen X that accepts calcium deposition is synthesized.
The Metaphysis
This zone is the site of vascularization, bone formation, and remodeling. The calcified matrix is removed, and fiber bone is formed and replaced by lamellar bone.
The Periphery
This zone includes the growth plate and metaphysis, which are the primary sites for infections, neoplasms, fractures, and metabolic and endocrine disorders. Problems in the growth plate constitute a significant portion of diseases of the musculoskeletal system in childhood.
Perichondral Ring
The perichondral ring of LaCroix and the ossification groove of the Ranvier ring surround the growth plate [B]. These structures support and expand the width of the growth plate.
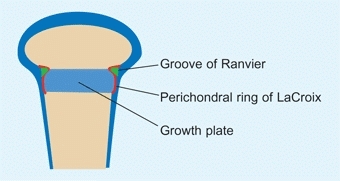
B Perichondral ring This ring consists of elements that provide strength and the capacity of the growth plate to expand in width. The groove of Ranvier (green) and perichondral ring of LaCroix (red) are shown. Based on Gamble, JG 1988.
The perichondral ring
of LaCroix is continuous with the metaphyseal periosteum, increasing the strength of the metaphyseal–physeal interphase.
The ossification groove
of Ranvier is an accumulation of chondrocytes, providing the reserve cells necessary for appositional growth of the growth plate.
Growth Plate Types
Growth plates form in a number of patterns, depending upon the shape of the bone [C]. These include:
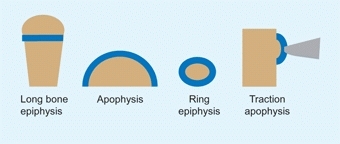
C Types of growth plates The common types include the long bone epiphysis that may be present at one or both ends. The apophysis is applied to the edge of a bone such as the iliac crest. Examples of ring epiphyses include the carpals or tarsal bones.
Epiphyseal plate
forms at the end of long bones, providing longitudinal growth.
Ring epiphyses
surround round bones such as the tarsals or metatarsals. The bones grow circumferentially.
Apophyses
are growth plates applied to the surface of a bone such as the iliac crest.
Traction apophyses
are growth plates to which a muscle is attached. Examples include the tibial tubercle and greater trochanter.
Bone Growth
Bone growth rate is precisely regulated with each growth center contributing a prescribed percentage of the final growth. These values are important to know due to their clinical significance [D].
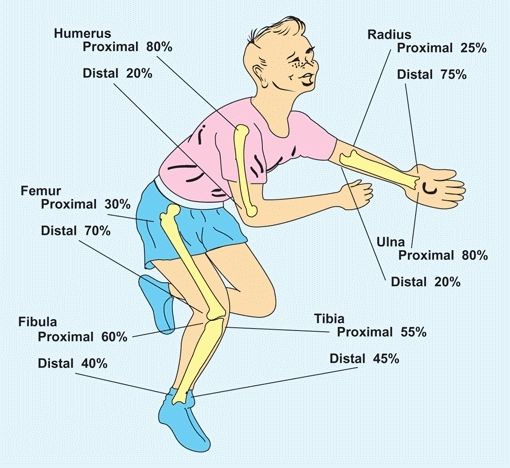
D Epiphyseal contribution of long bone growth From Blount (1955).
Growth rates from various epiphyses vary. In the upper limb, growth is most rapid at the shoulder and wrist, in contrast to the lower limb where most growth occurs just above and below the knee. To help remember the different growth rates in extremities, a commonly used memory aid is imaging a child in a bathtub. The growth plates that would be above water are the most fast growing [E]. Alterations in the general health of the child are often recorded by growth arrest lines [A]. These are linear calcifications from slowing of growth.
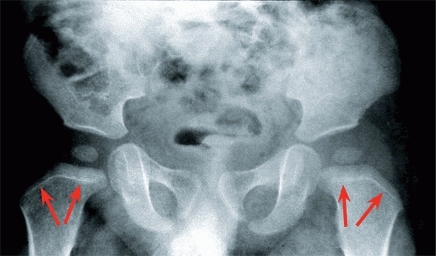
A Growth lines Note the growth arrest lines (red arrows) in this child with developmental hip dysplasia. Presumably, the anesthetic and closed reduction caused the slowing of growth. Bone growth since the event is shown by the width of the new metaphyseal bone.
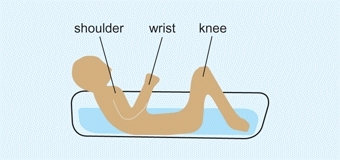
E Growth memory aid Child in a tub. The growth centers around the shoulder, wrist, and knee (the parts out of water in this drawing) are the fastest-growing centers in the limbs.
Growth and Development
Infancy extends from birth to 2 years of age. It encompasses the period of most rapid growth and development after birth.
Growth Rates
The growth rate varies with age and is greatest in early infancy, declines during childhood, and briefly increases again during the adolescent growth spurt. A child is about half his or her adult height at 2 years of age and about three-fourths by 9 years of age [A].
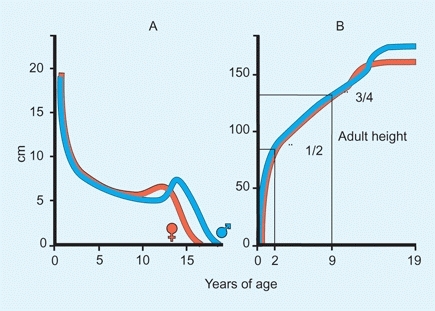
A Growth Rate A Growth rates for girls (red) and boys (blue) by age. The greatest rate of growth occurs during infancy. B Growth rate as a fraction of adult height. About half of an individual’s adult height is reached by age 2 years and three-fourths by age 9 years.
Predicting Adult Height
Estimating adult height is valuable in managing certain deformities, particularly anisomelia (limb length inequality). A variety of methods for predicting adult height are available.
The simplest method is to double the child’s height at two years [A].
Another way involves establishing the percentile of height by plotting the child’s height on the growth chart by bone age rather than by chronological age. This percentile is projected out to skeletal maturity to provide an estimate of adult height [B].
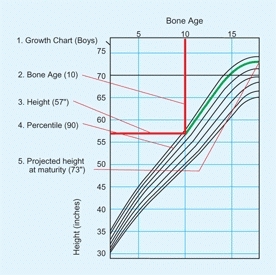
B Prediction of adult height Predict adult height by plotting the child’s bone age (vertical red line) against the current height (horizontal red line) to determine the percentile value (green). Follow the percentile (green line) to skeletal maturation to estimate final adult height.
The most commonly used method [C] is to add the mother’s and father’s heights in either inches or centimeters. Add 5 in. (13 cm) for boys and subtract 5 in. (13 cm) for girls. Then divide in two. Using this method, most children will reach an adult height within 4 in. (10 cm) of this estimation.
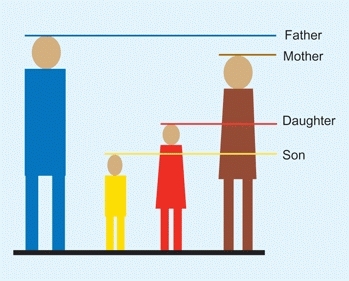
C To estimate adult height Add heights of father and mother. For daughters subtract 5 in. (13 cm) and divide in two. For sons add 5 in. (13 cm) and divide in two.
Growth of Different Tissues
The growth rate of tissues varies with age. Subcutaneous fat, which provides nutritional reserve and protection from cold and injury, develops during the first year. The fat obscures the longitudinal arch of the foot, giving the infant a flatfooted appearance [D]. The percentage of muscle increases with age, but the percentage of neural tissue declines with advancing age.
D Subcutaneous fat in infancy Note the thickness in the subcutaneous fat (arrows) in this infant undergoing clubfoot correction.
Body Proportions
Growth of various body parts are different from one another. Upper limb growth occurs earlier than lower limb growth, and the foot grows earlier than the rest of the lower limb. In childhood, the trunk grows most rapidly; in adolescence, the lower limbs grow the fastest. Throughout growth, body proportions gradually assume adult form [A].
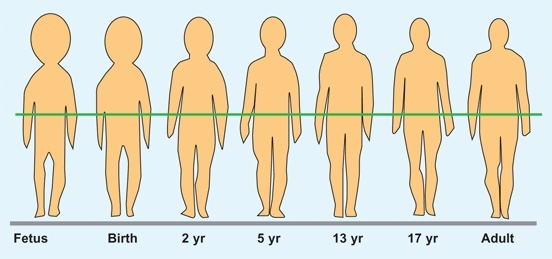
A Changes in body proportions with growth At maturity, the position of the center of gravity (green line) is the level of the sacrum. From Palmer (1944).
Determining Maturation Level
Knowing the amount of growth remaining is important to the timing of physeal fusion and thus in correcting leg length inequality [B] and in managing patients with scoliosis.

B Leg length inequality Bone age determination is helpful in planning correction by epiphysiodesis.
Hand–wrist radiographs
Use the Greulich–Pyle atlas to estimate the bone age.
Risser sign
is based on the extent of ossification of the iliac crest as assessed on the AP radiograph [C]. This sign has been commonly used in assessing maturity when managing scoliosis.
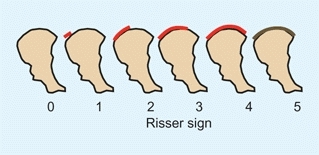
C Risser sign The extent of ossification of the iliac apophysis is commonly used to assess the skeletal maturation of patients with scoliosis. Risser 0 = no iliac apophysis; Risser 5 = fusion of the apophysis with the ilium.
Tanner stages
The level of maturation is based on physical examination. Because this assessment requires an assessment of breast and genital development [D] in a sensitive age group, its use is limited.
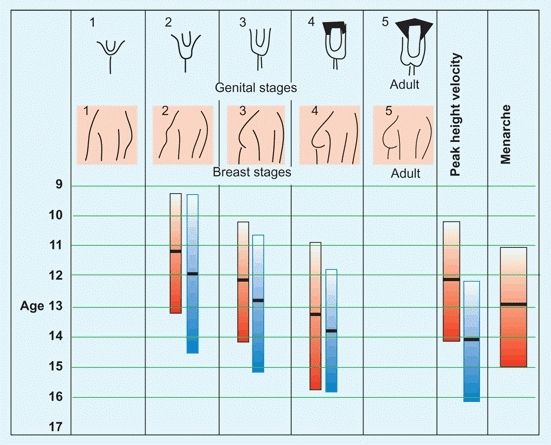
D Tanner maturation index Using physical signs, the level of maturation is assessed for males (blue) and females (red). The columns show the 3–97% levels. Mean values are shown by the black bars.
Other signs,
such as the velocity of height gain and the status of the triradiate cartilage (acetabulum), are becoming useful maturational indices.
Extremes in Growth
Wide ranges of normal exist in normal individuals [A]. However, orthopedic problems are more likely to occur in excessively heavy or short children. We define excessive as being either above the 95th percentile in weight or below the 5th percentile in height [B].

A Growth variations These individuals show the wide variations in growth. Courtesy of Dr Judy Hall.
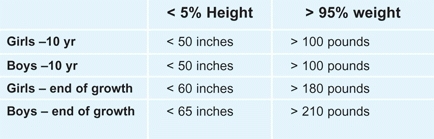
B Extremes of height and weight Orthopedic problems are more common in children who are excessively short (below the 5th percentile) or excessively heavy (above the 95th percentile). These values are given for girls and boys based on North American standards.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








