Animal
Gene
Scaffold
Vector
Outcome
Reference
No carrier
Rat
BMP-2
Triacrylate/amine-gelatin
Free plasmid
Minimal to no effect on bone formation
Chew et al. [19]
Synthetic carrier
Rat
PDGF-B
Collagen
PEI
14- and 44-fold higher new bone volume/total volume in treated calvarial defects compared to empty defects or empty scaffolds after 4 weeks of implantation
Elangovan et al. [27]
Rabbit
BMP-2
Porous HA
Cationic liposomes (SuperFect™, QIAGEN GmbH)
Robust bone formation in the cranial defect in animals treated with BMP-2 gene without HA; complete ossification observed at 9 weeks
Ono et al. [83]
Mouse
caALK6 and Runx2
PEG-block catiomer
Linear PEI (Fermentas) and FuGENE6 (Roche)
Bone formation covering entire lower surface of the implant at 4 weeks
Itaka et al. [50]
Rat
BMP-2
PFF
TAPP complexed with GMP
No enhanced bone formation (via micro-CT and histology) at 12 weeks postimplantation
Chew et al. [19]
Rat
BMP-4
PLGA
PEI
Enhanced bone regeneration at defect edges measured via histological and micro-CT evaluation; increase in osteoid and mineralized tissue density
Huang et al. [48]
Synthetic Carriers Used in Gene Delivery
Gene therapies are intended to maintain optimal doses and local concentration of therapeutic proteins over a period of time under a minimal side effect which demonstrates the superiority of gene delivery over conventional protein delivery [81]. Significant achievements in gene delivery for bone regeneration and their intraosseous expression using both viral vectors, derived from adenovirus, retrovirus, lentivirus, and synthetic vectors, liposomes, and cationic polymers/dendrimers, have been reported elsewhere [4, 6, 40, 49]. Both ex vivo and in vivo approaches to delivery have been attempted with both types of vectors (Fig. 20.1) [92].
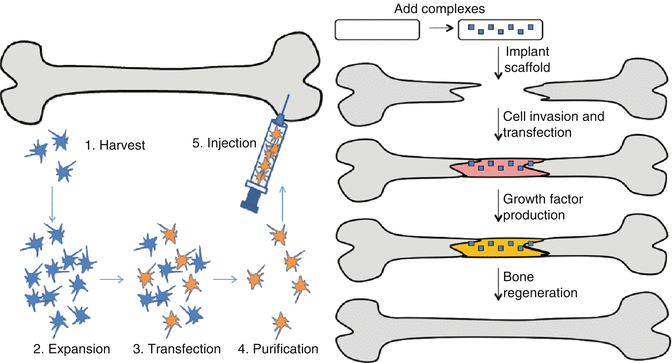

Fig. 20.1
Gene delivery approaches to the bone. In vivo approach (right) relies on the introduction of the therapeutic genes directly in suspension form or via gene-activated matrix (as shown). Ex vivo approach (left) relies on modified primary cells and subsequent implantation to the repair site (Adapted from Rose et al. [92] with permission)
Nonviral gene delivery has been recently emphasized in the field due to facile chemical strategies, stability for long-term storage and reconstitution, safe toxicity profiles, and unlimited capacity of gene sizes (cargo). In gene therapy of the bone, two fundamental (indispensible) aspects of the therapy are carrier vectors (i.e., cationic molecules with enough binding capacity to protect nucleic acids and facilitate intracellular trafficking) and biomaterial scaffolds (i.e., a three-dimensional construct to deliver complexes to repair sites, control release kinetics of complexes, and provide a milieu for osteoinduction). Effective nonviral vectors are generally constructed from cationic polymers, dendrimers, or lipids, but cationic polymers are the most attractive candidate [78, 80, 85, 89]. The common cationic polymers employed in these approaches comprise of polyethylenimine (PEI), poly(L-lysine) (PLL), poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA), poly(amidoamine) (PAMAM), and chitosan (CS) (Fig. 20.2a) [78, 80, 85].
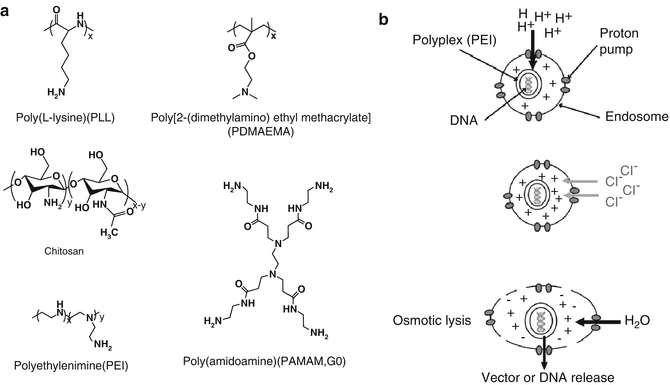

Fig. 20.2
(a) Chemical structure of common synthetic cationic polymers/dendrimers used in nonviral gene delivery. (b) Schematic representation of proton-sponge effect of the cationic polyplexes (The figure is adapted from Ref. [80] with permission)
Electrostatic interaction between cationic primary amines of polymers and anionic phosphate of polynucleotides forms the foundation of nonviral gene delivery. It leads to formation of condensed polyionic complexes (polyplexes) and protects the encapsulated cargo from enzymatic and nonenzymatic degradation, avoids the clearance through the reticuloendothelial system (RES), enhances cellular uptake via interactions with anionic cell surface proteoglycans, and finally increases half-life in the cytoplasm [3, 24, 99]. Many studies have shown that factors such as size, surface charge, chemical composition, degradability, and stimulus sensitivity affect cellular uptake and intracellular trafficking [17, 56]. The widely accepted benefit of cationic polymers in gene delivery is thought to be derived from their extraordinary cationic charge density and buffering capacity. Buffering capacity is a specific feature of cationic polymers that enables “proton-sponge” effect (Fig. 20.2b) [108]. In PEIs particularly, secondary and tertiary amines generate buffering capacity over a wide range of pH values and facilitate endosomal escape. It has been reported that the gene delivery efficiency of cationic polymers depends on degree of polymerization (molecular weight), branching (topology), and the buffering capacity, which is a function of cationic charge density. As an example, Godbey et al. reported that, under in vitro condition, transfection efficiency of PEIs increases with molecular weight (MW) (70 kDa PEI >10 kDa PEI >1.8 kDa PEI) [42]. However, in vivo efficiency decreases with MW (25 kDa PEI >50 kDa PEI >800 kDa PEI) [1]. Despite intensive activity, however, concrete relationships among structure-property-functional performance remain incompletely described [37]. In the last few years, PEIs and its derivatives are investigated in gene delivery for bone regeneration studies in both ex vivo and in vivo models [27, 93]. The efficacy of native polymers is generally improved by hydrophobic modification using aliphatic lipid molecules [93]. The effect of MW in transfection efficiency was also observed in PLL, a widely used biodegradable polypeptide. Low MW PLL (<3 kDa) cannot even form complexes with DNA, whereas the efficacy of PLL 211 kDa/DNA complexes was 20-fold higher than PEI-20 kDa/DNA complexes, but the intolerable toxicity of higher molecular weight PLL limits its frequent application [64]. The amines of PLLs are completely protonated at physiological pH indicating inefficient buffering capacity, an essential mechanism for endosomal escape [2]. Dendrimers are another class of synthetic polymers with spherical highly branched geometry that comprises of primary amines on the surface to participate in DNA binding and buried tertiary amines to generate the proton-sponge effect. The particular interest in PAMAM is due to their customizable structure with reasonable functionality, which provides enough space for tailoring of appropriate ligand [23]. Like linear polymers, transfection efficacy of PAMAM dendrimers is also proportional to MW (i.e., the generation number) [61]. The CS, on the other hand, is a natural cationic polysaccharide polymer with a good biocompatibility and mucoadhesive and immunogenic properties that are obtained by partial deacetylation of chitin derived from crustacean shells [7].
Calcium phosphate (CaP) is one of the most studied inorganic materials employed to fabricate gene-activated matrix (GAM) for bone tissue engineering [9, 62, 113]. CaP/DNA coprecipitation technique has been used since 1970 for in vitro gene delivery due to its simplicity and nontoxic profiles [22]. CaP complexes of DNAs are tight and compact that likely keep DNA intact at transplanted or injected site; this increases its bioavailability, which is greater than common polymeric carriers [34]. These complexes display enough resistance against serum DNases that is the cause for higher efficacy [62].
In recent years, multiple strategies are being actively pursued in designing second-generation polymeric carriers via chemical modification [56, 118] and by engineering prefabricated nanostructured carriers [101]. Chemical modification alters the physicochemical properties of native polymers and generates new chemical functionalities that can further alter the physical and biological properties of native polymers. Despite extensive work in cell culture, the potential of the vast array of second-generation polymers remains to be fully explored in bone regeneration.
Gene-Activated Matrices (GAMs) for Gene Delivery
Controlled release of an expression vector (pDNA) to surrounding tissues can be achieved by a GAM. Diverse materials such as collagen, CS, silk, synthetic polymers, minerals, and their composites can be utilized as the basis of GAM. The specific examples from published studies were provided below. For enhancing GAM’s transfection effectiveness in cells and triggering more effective bone regeneration in vivo, researchers have utilized PEI [48], CaP precipitates [28], or liposomes [74] as the pDNA carriers, although pDNA without any carriers were also attempted [94].
In a recent study, collagen scaffolds were used to deliver PEI/pDNA complexes encoding PDGF-B for bone regeneration in a rat calvarial model [27]. Bone bridges were established at critical-sized defects in rats that were healed with scaffolds involving PEI/pDNA complexes alone. Trabecular bone volume fraction, degree of trabecular connectivity, and connectivity density were significantly higher in complex-enclosed scaffolds than the control interventions. A collagen sponge incorporating pDNA encoding parathyroid hormone (PTH) 1–34 or BMP-4 was explored by Fang et al. [31]. Osteogenic stimulation was obtained in segmental defect models in rats with desired consequences. In another study, the osteogenic potential of BMP-2 gene/fibronectin/apatite composite layer on hydroxyapatite (HA) ceramic scaffolds was investigated. The scaffolds were implanted into rats subcutaneously [110], and gene expressions for BMP-2 and alkaline phosphatase (ALP) were found to be increased upon application of composite layer-coated scaffolds. Administration of PEI-condensed pDNA encoding for BMP-4 from poly(lactic-co-glycolic acid) scaffolds was studied in rat cranial critical-sized defect models [48]. Bone regeneration was significantly enhanced at defect edges according to histological and microcomputed tomography evaluation in this approach. Increase in osteoid and mineralized tissue density was also obtained. Bone defects in a very large animal (horses) were repaired with collagen matrix containing human PTH (1–34) expression vector [5]. Enhanced bone formation in cortical defects was observed in human PTH-collagen matrix group.
Most GAM explored the delivery of a BMP-2 expression vector by using a carrier (or transfection agent). HA ceramic buttons with a layer containing BMP-2 gene and fibronectin were utilized to treat bone defects on cranium of rats [116]. Results showed that expressions of BMP-2, ALP activity, and osteocalcin were elevated in the HA-BMP-fibronectin group, with increased bone formation. An alginate hydrogel containing BMP-2 expression vector and goat multipotent stromal cells was implanted intramuscularly in goats [112]. Bone induction was observed at the implanted ectopic sites, and goat multipotent stromal cells/BMP-2 pDNA treatment resulted with higher collagen deposition and bone formation. A BMP-2 expression vector was also implanted in dorsal muscles of rats by using HA fibers [82]. The HA fibers incorporating high doses (50 or 100 μg) of BMP-2 expression vector induced more bone mineral content than other implant groups at 4 weeks. The HA fiber containing 50 μg dose of BMP-2 expression vector led to higher osteogenesis as compared to other groups according to radiographic analyses at 8 and 12 weeks. Alternatively, a pDNA encoding BMP-2 in triacrylate/amine polycationic polymer (TAPP) was applied with gelatin microparticles buried within scaffolds in a critical-sized rat cranial defect model [19]. Unlike other studies, the TAPP/pDNA polyplexes did not cause higher bone formation rate than other groups. The reason for this lack of effect could be due to polycationic polymers that had slow degradation rate to trigger sustained pDNA release from scaffolds. Also, in situ cytotoxicity of the polymers might have prevented bone induction, which should be evaluated in conjunction with osteogenesis studies. Bovine atelocollagen and pDNA that encodes human BMP-2 with CaP combination were investigated for treatment of critical-sized segmental bone defects in rats [28]. Bone defects were started to be healed and improved bone strength was obtained in BMP2/CaP-collagen group. Finally, a unique gene formulation was developed by coating preformed cationic PEI/pDNA polyplex with anionic peptide-PEG copolymers for development of so-called copolymer-protected gene vector (COPROG) [100]. Kirschner wires were then coated with COPROG/BMP-2 pDNA and applied in rat tibias intramedullary. Based on biomechanical analysis, the highest load was obtained with the BMP-2 gene delivery group, indicating the feasibility of turning a fracture stabilization device into a plasmid (gene) delivery system.
In addition to these osteogenic genes that were intended to directly stimulate osteogenic events, genes for cytokines involved in angiogenesis were also delivered for indirect stimulation of bone induction. A pDNA coding for human VEGF-165 was coated on collagen sponges (using no carriers or transfection agents) and applied to critical-sized defects in rabbits [38]. More bone formation and endothelial area were observed in the VEGF gene-delivered group, presumably linking enhancing angiogenic activity to bone deposition ultimately.
The synthetic siRNA has been alternatively used to silence specific targets and stimulate bone formation. Dioleoyl trimethylammonium propane (DOTAP)-based cationic liposomes were used in rats to deliver a siRNA that targeted casein kinase-2 interacting protein-1 (encoded by Plekho1) [117]. The mass (given by bone mineral density) and micro-architecture of bone were augmented in treated groups. Implantation of silk fibroin-CS scaffolds with a siRNA against guanine nucleotide-binding protein alpha-stimulating activity polypeptide 1 and prolyl hydroxylase domain-containing protein 2 was studied in the periosteum of sheep [91]. An increase in induced bone volumes was observed in the siRNA treatment groups.
In other studies, transfected cells/scaffold combinations were investigated which functions differently from the principle of GAMs. The basic fibroblast growth factor (bFGF)-transfected MSC containing beta-tricalcium phosphate ceramics (beta-TCP) were applied to critical-sized segmental bone defects of rabbits [44]. The results demonstrated that capillary-bone regeneration was higher in bFGF-transfected MSC/beta-TCP group. Arthrodesis at the dorsal spine of rats was treated with devitalized bone matrix which was soaked with bone marrow cells [8]. In a controlled design, one defect site had marrow cells transfected with complementary DNA (cDNA) encoding LIM mineralization protein-1 (LMP-1), and the other site had cells transfected with a reverse copy of the cDNA (as a control). Spine fusion and bone formation were significantly induced by marrow cells transfected with LMP-1 group, but not in the control group.
Direct Injection of Genes and Gene-Modified Cells
Direct injection of expression vectors to wound sites was proposed as a simple, clinically convenient way for bone induction and repair [73]. A significant concern with direct injection approach is the dissemination of the expression vectors to neighboring tissues and haphazard ossification [87]. The optimal timing of injection needs to be evaluated for each gene, that is, the timing of injection after a bone defect might be evaluated. The persistence of gene expression might be additionally difficult to control, but these are important variables to understand for an efficacious therapy [54]. Examples of studies that employed direct injection of pDNA or cells modified with therapeutic genes are below.
A pDNA encoding for osteogenic protein-1 (OP-1; BMP-7) with a collagen carrier was injected to rats as a model for posterolateral lumbar interbody arthrodesis [11]. Based on the radiological and histological results, bone formation was evident in pDNA/collagen-applied groups. A calcium phosphate/pDNA nanoparticle formulation encoding for BMP-2 was also found to be functional when injected subcutaneously in alginate hydrogels in mice [60]; bone formation was evident when pre-osteoblast cells were injected along with such a BMP-2 gene expression system. Beyond the delivery of BMP genes, the effect of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) gene delivery on osteosarcoma and Ewing’s sarcoma was investigated in mice [88]. Bone fractures are usual consequences of tumor expansion and broad bone disruption of Ewing’s sarcoma [66], so that gene therapy could be beneficial at sites of localized, excessive bone resorption. Lipophosphoramide/DNA injections were undertaken as TRAIL therapy into retro-orbital veins of osteosarcoma and Ewing’s sarcoma models. Overexpression of TRAIL was confirmed in plasmid construct-applied groups, and tumor incidence and osteolytic lesions were decreased in TRAIL-applied groups.
Gene-modified cells were also applied directly for bone regeneration. Healing of bone defects in the rabbit tibia was explored with fibroblasts that were transfected with VEGF [67]. More ossification as bone bridges and vessel formation were observed in VEGF fibroblast group. Bone marrow-derived MSCs (BM-MSCs) that were transfected with osterix (OSX) gene were also injected to distraction gaps in the mandibles of rabbits [65]. Increased bone development in distracted callus and also higher bone sialoprotein expression were observed in OSX-modified BM-MSCs treatment groups.
Electroporation and Sonoporation in Gene Expression
Utilization of electrical pulses for creating pores across plasma membrane is the process of electroporation [97], which can enable pDNA transfer into a cell without the need for a carrier. The operational parameters of pulses are important for effectiveness of transfection in this approach. Similarly, ultrasound-applied gene transfer (sonoporation) can serve for the same end by relying on microbubble-induced cell permeabilization for pDNA uptake [67, 68, 76]. Easier clinical translation and decreased invasiveness of sonoporation make it superior to electroporation [15]. Assimilating pDNA by electroporation or sonoporation percutaneously can be hard because of restriction by dense tissues enclosing human bones [94]. Studies involving electroporation and sonoporation showed that bone formation could occur especially in ectopic sites of bone in mice and rats following gene delivery [51–53, 57, 59, 84, 103]. A collagen sponge with pDNA encoding BMP-9 was injected into nonunion fractures of mice in one study [55]. After implantation, electroporation was undertaken and bone bridging was observed in electroporated BMP-9 group by histological analysis and microcomputed tomography. Feichtinger et al. investigated injection of BMP2/7 co-expressing pDNA with ultrasound application transcutaneously for bone regeneration in mice and rats [32]. Assessment of gene transfer effectiveness was performed with bioluminescence activity, which resulted with success rates of 85 % at 2 W/cm2 and 100 % at 4 W/cm2. In addition, sonoporation caused higher bone regeneration percentages according to microcomputed tomography consequences.
Perspective
Obtaining convenient means for harmless and effective gene delivery is difficult in functional bone regeneration, despite the availability of a spectrum of delivery agents. The spectrum of carriers functional for gene delivery is encouraging, but concerted efforts to understand and overcome factors limiting gene expression are continuously needed. The biocompatibility and mechanical properties of materials need to be evaluated on one hand [111], while the in situ residence of pDNA (or other expression vectors) at the defect site needs to be ensured in a functional state [60]. Animal models are essential to explore the proof of principle for the newly developed therapies, but investigation of therapeutic features beyond functional outcomes (e.g., biocompatibility, immune response, etc.) must be also incorporated in such studies. Minimal immunogenicity associated with nonviral approaches of gene delivery makes it safer than the viral approaches of gene delivery [14]. Delivering growth factors in gene form may become more practical over utilization of high doses of protein, to better sustain protein presence at the site [104] and possibly overcome adverse effects associated with high protein doses locally. Since most clinical cases requiring bone repair are non-life threatening, it is likely that the nonviral approach will gain the upper hand on the long run (i.e., viral delivery is difficult to justify in such clinical scenarios). Studies in larger animal models (as compared to rodents) will be required to better realize the potential of gene-induced bone repair in slower-growing or nongrowing organisms reminiscent of humans.
Acknowledgments
The studies in the author’s lab were supported by operating grants from NSERC and CIHR and infrastructure grants from Alberta Innovates – Health Solutions.
References
1.
Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA (1996) A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther 7(16):1947–1954PubMed
2.
Akinc A, Langer R (2002) Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal trafficking. Biotechnol Bioeng 78(5):503–508PubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








