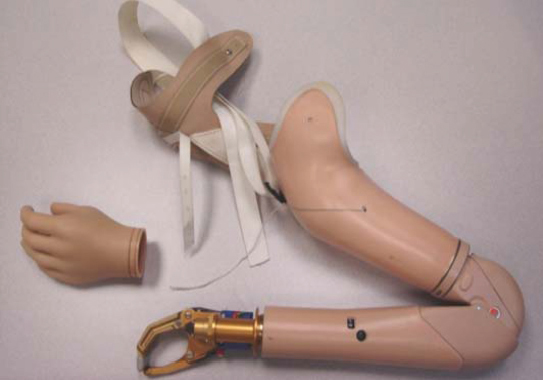
49 Key Points 1. Neuroprosthetic devices are helping to restore lost motor or sensory function as a result of aging, disease, or injury. They do so by acting as a bridge between functioning elements of the nervous system and dysfunctional limbs, prosthetic devices, damaged nerves, or receptor organs. 2. Functional electrical stimulation and brain–machine interfaces have helped promote the restoration of lost neural function and improve quality of life by interfacing with neuroprosthetic or robotic devices to allow the individual to regain some of the lost function. 3. Targeted reinnervation is a technique that can provide more natural control of prostheses than functional electrical stimulation and bypass brain–machine interface problems related to degradation from the body’s natural immune response. This technique can provide both motor control and sensory feedback and can interface with new robotic prostheses or existing commercially available myoelectric prostheses, such as powered wrists or elbows. Advances in robots and their control provide tremendous opportunities for restoring function to disabled patients. Over the past few decades, research in neuroscience and engineering and the merging of these two fields have enabled this. In engineering, remarkable end terminal devices, such as prosthetic hands, are the direct result of application of new materials, innovative designs, and on-board microprocessing. In neuroscience, there are new insights in how motor function and sensory perception are cortically controlled from innovations for extracting signals from both central and peripheral nervous system sources and algorithms for decoding these signals. Together, these achievements are enabling revolutionary ways of transforming user intent into user actions. Disability is common. By far, the leading cause of disability is age-related frailty. The loss of either motor or sensory function can also be a result of traumatic brain and spinal cord injuries, amputations, and strokes. Currently, more than 200,000 patients in the United States have been involved in traumatic spinal cord injuries, with almost half of these patients being paralyzed below the neck. There are also more than 5,000,000 stroke survivors and 400,000 amputees living in the United States.1 Emerging techniques using neural control, such as brain–machine interfaces (BMIs) with robotic devices, such as an exoskeleton or prosthetic limb, hold promise to more effectively restore a person’s function than was previously thought possible. The field of neuroprostheses focuses on technology to restore function through neural control. This is the critical feature of a neuroprosthesis and what clearly distinguishes it from a traditional prosthesis. The neuroprosthesis is controlled in ways that allow the patient to think naturally about an intended action and thereby to actuate the prosthesis to do that specific action (e.g., think about wiggling a finger and a robot finger wiggles, or think about moving a computer cursor left and the cursor moves left). This is distinct from how a traditional prosthesis (Fig. 49.1) is controlled using the myoelectric approach. Myoelectric control uses residual muscles to control artificial end terminal devices. To accomplish an intended action, a patient must think indirectly to actuate the device. For example, a prosthetic hook hand that is controlled by the biceps would require the patient to imagine flexing a forearm to open the hook hand such that a pencil could be grasped. This is clearly different than simply thinking about opening one’s hand. Such indirect thinking makes even more complex actions, such as touch typing a keyboard, extremely difficult to achieve. So, by using the patient’s natural intent, a neuroprosthesis can achieve levels of functionality far greater than that of a traditional prosthesis and likely to the same level of the native limb. To use direct neural control, a neuroprosthesis must interact directly with the patient’s nervous system. The site of interaction depends on where the lesion is and would need to be above the level of that disruption. Neuroprostheses can intercalate at any level of the nervous system from cortical gray to spinal anterior horn to peripheral nerve to neuromuscular junction to muscle. The approaches are varied and include peripheral ones, such as functional electrical stimulation (FES) and targeted reinnervation, and central ones, such as penetrating cerebral cortical electrodes and electrocorticography (ECoG). They also vary in terms of invasiveness. Surface electromyographic (EMG) and electroencephalographic (EEG) devices are virtually noninvasive, whereas cortical penetrating electrodes requiring craniotomy are invasive. Fig. 49.1 Traditional upper extremity prosthesis that requires residual muscle actuation to provide basic control of the device, with limited pincer and elbow flexion function. The development of neuroprostheses began with devices that electrically stimulated peripheral nerves or muscle. This was initially achieved using surface electrodes placed directly over muscles (surface EMG), or in close proximity to motor nerves (surface ENG). Eventually, development advanced to the level that some neuroprostheses functioned through direct cerebral cortical control. It was originally proposed that direct interfaces between spared cortical or subcortical motor centers and artificial actuators could be employed to bypass spinal cord injury. This research resulted in the early development of BMI.2 BMIs are devices that work in direct communication with the brain. The electrical signals used for actuation are derived from EEG, ECoG, or intra-cortical monitoring electrodes. Electrodes can be microwires placed individually or in arrays. The Utah Electrode Array (UEA) is a commonly used device, named by the researchers at the University of Utah and University of Michigan who developed it.3 The UEA is silicon 4 mm × 4 mm platform with 100 1.5 mm long microelectrodes designed to be placed directly onto the surface of the brain with the microelectrodes penetrating into brain parenchyma. It can also be placed on a peripheral nerve. Initial research focused on motor prosthesis control, but continued advancements have led to the development of sensory prosthetic devices as well. Sensory prostheses record and process inputs from the environment and afferently transmit this information back to the appropriate cerebral sensory cortex. Notable examples of sensory prostheses include retinal implants for vision restoration and cochlear implants for hearing restoration. Implantable neuroprostheses for motor rehabilitation have been explored since the 1960s. Motor prostheses were devised that electrically directly stimulate muscles or motor nerves. Early technology was based almost exclusively on FES, an efferent approach. However, as robotic prosthetic limbs became more sophisticated (Fig. 49.2), other component technologies were developed that allowed for more complete integration. Afferent approaches are now included, such as biosensors to detect signals from the user’s sensory nerves, muscles, or joints. Such information is relayed to a controller located inside the device, and together with feedback from the limb or actuator, action can be regulated more efficiently and precisely. Presently, intracortical BMIs are being developed to interface with the sensory cortex and thalamus with end terminal devices. Because developing BMI systems is very complex and time consuming, beginning in 2000, the Brain–Computer Interface R&D Program at the Wadsworth Center of the New York State Department of Health began to develop a general purpose system for BMI research known as BCI2000.4 The BCI2000 project facilitates research and development in the areas of data acquisition, stimulus presentation, and brain monitoring applications. The vision of the BCI2000 is that it will become a widely used software tool for diverse areas of real-time biosignal processing. It is currently free for nonprofit research and educational purposes and has been provided to over 400 laboratories worldwide. FES uses electrical stimulation to effect neuromuscular transmission peripherally by activating nerves that innervate paralyzed extremities. Initial studies of restoring movement to paralyzed limbs with electrical stimulation date as far back as the mid-19th century.5 The first truly modern FES device was developed in 1961. This early prototype used surface electrodes that activated the peroneal nerve to treat footdrop.6 A decade later, implanted peroneal nerve stimulators to treat hemiplegic foot drop were introduced.7,8 Also in the 1960s, radio frequency–controlled stimulators of the detrusor muscle in the bladder were implanted in a select number of incontinent patients.9,10 In 1979, Brindley and colleagues implanted five paraplegic patients with sacral anterior root stimulators, all of whom were able to void when stimulated.11 This led to larger human trials in the 1980s, in which similar devices controlled by an external transmitter were successfully implanted over the sacral ganglia. These devices would deliver intermittent stimulation, resulting in improved bladder emptying. They would also assist in defecation and restore the ability to sustain a full erection in male patients. Fig. 49.2 New-generation robotic arm developed at Johns Hopkins Advanced Physics Laboratory. This arm can currently be used with external and indirect neural controls. Work is ongoing to develop brain–machine interface techniques for eventual direct neural control. In 1991, BIOnic Neurons (BIONs) were developed. These are single-channel micromodule implants that are about the size of a grain of rice. Power and digital command signals are from inductive coils, which are positioned near or worn over the implant.12 BIONs are used clinically for therapeutic muscle stimulation to electrically exercise paralyzed and weak muscles to prevent or reverse disuse atrophy. BIONs have gone through four generational iterations, all of which are designed to stimulate myelinated sensory or motor axons, typically in peripheral nerves. BION1 and BION2 require an external radio frequency–powered transmission coil for power and programming. An advantage of BION2 is that it allows for two-way signal transmission (stimulation and recording) through bidirectional telemetry. BION3 devices are focused on eliminating the continuous dependence of the implant on external coils for powering and control. A BION3 still requires an external transmission coil for programming and charging; however, downloaded stimulation paradigms can be implemented autonomously with power supplied through an on-board rechargeable battery. Finally, BION4 devices incorporate a rechargeable battery power similar to the BION3 but have a high-rate communication protocol that allows large numbers of implants to exchange information freely among themselves and with an external controller.13 In 1986, the Neurocontrol Freehand Stimulator (NFS), a surgically implanted FES device, was developed by the Cleveland FES Center at Case Western University. This is a radio frequency–powered motor neuroprosthesis that utilizes low levels of electrical current to stimulate peripheral nerves that innervate muscles in the forearm and hand to provide functional hand grasp patterns. The NFS involves implantation of up to eight electrodes in the muscles of the forearm and hand. Typically the NFS is implanted into the brachioradialis and extensor carpi radialis for voluntary wrist extension, and the posterior deltoid to triceps is used for elbow extension. The electrode wires are tunneled up the arm to a control box located under the skin in the pectoral region, and a movement detector is placed externally on the opposite shoulder. Opposite shoulder movement is then relayed to the control box, which is programmed to coordinate the activity in the electrodes and cause the hand to open and close. The device received US Food and Drug Administration (FDA) approval in 1997, and over 250 C5 and C6 quadriplegic patients have been successfully treated since that time.14,15 Design improvements in FES are under way to fine tune motor movement and to use implanted rechargeable power sources and wireless telemetry.16 The limitations of FES devices are that they rely on indirect control because movement of underlying muscle activation from a nonparalyzed body part is used to trigger the coordinated electrical stimulation of muscles in the paralyzed limb. In the 1970s, early BMI work began at the University of California, Los Angeles (UCLA)17 with support from the National Science Foundation and the Defense Advanced Research Projects Agency (DARPA). A BMI is simply a device that can decode human intent from brain cortical activity. By doing so, an alternate communication channel for people with severe motor impairments is created. Studies as early as the 1960s demonstrated that nonhuman primates could learn to voluntarily control the firing rate of primary motor cortex neurons using operant conditioning.18–20 Algorithms were developed using motor cortex electrical signals. Such algorithms allow activity from motor cortex neurons to be made into intent. In the 1980s, Georgopoulos and his research team at Johns Hopkins University found a mathematical relationship between the electrical responses of single motor cortex neurons in rhesus macaque monkeys and the direction that the monkeys moved their arms. The research team also demonstrated that dispersed groups of neurons in different areas of the brain collectively controlled motor function. However, they were only able to record the firings of neurons in one area at a time because of the technical limitations imposed by their equipment.21 Since then, rapid advances have occurred, with several groups being able to capture complex brain motor signals from groups of neurons to control external devices.22 Numerous studies and extensive research have been performed in regard to neural recording methods for BMI, such as microelectrodes, local field potentials, magnetic resonance imaging (MRI), and ECoG.23–25 It has been shown that nonhuman primates can control robotic arms and hands using cortical control. Nicolelis at Duke University demonstrated cortical control of a computer cursor by monkeys with cortically implanted microwires. He then showed that these monkeys were able to control a robotic neuroprosthetic arm to move objects around a board.26,27 Schwartz at the University of Pittsburgh then showed that nonhuman primates with cortically implanted UEAs can reach, grasp, and feed themselves food rewards using a cortically controlled neuroprosthetic upper extremity.28 Hwang and Andersen at CalTech demonstrated that, by using signals from the posterior parietal cortex, neuroprosthetics can be controlled in near real time.29 Schieber at the University of Rochester has shown monkeys can use cortical control to actuate individual fingers of a neuroprosthetic hand.30 All of these investigators have shown what is possible with direct cortical control. Initially, studies on BMIs were based on scalp electroencephalograms (EEGs) due to their noninvasive nature and easy applications.23 These EEG-based systems were developed as communication aids, such as moving a computer cursor or performing keyboard inputs, but were limited to these types of applications.31,32 Scalp EEG recordings lack the spatial and temporal resolution that is required for dexterous control of a robot arm in real time due to reflecting electrical activity of millions of neurons in widespread areas of the cortex. Although these devices are easy to wear, the signals are suboptimal because of the skull dampening. To improve spatial resolution, MEG and functional magnetic resonance imaging (fMRI) were explored, but these applications required special equipment that is impractical for common everyday use.25,33 In the 1980s, an EEG-based BMI was developed that used the P300 brainwave response to allow the subject to communicate words, letters, and simple commands to a computer that converted the data through a speech synthesizer.34 In the 1990s, Birbaumer from the University of Tübingen in Germany used EEG recordings of cortical potentials to give paralyzed patients limited control over a computer cursor. Ten patients were trained to move a computer cursor with these signals. The disadvantage was that the process was slow, training took many months, and, even after becoming expert with the system, patients required over an hour to assemble just 100 characters. In 1999, Peckham and his team at Case Western Reserve University used a 64-electrode EEG skullcap to provide limited hand movement to a quadriplegic patient. Mr. Jatich was instructed to concentrate on simple but opposite concepts like up and down, while his β-rhythm EEG output was analyzed. When a basic pattern was identified, it was used to control a switch, which later enabled the patient to control a computer cursor to drive nerve controllers embedded in his hands, restoring some movement. As before, the limited data that could be obtained by this method meant significant patient training to gain only rudimentary function. Because of the limitations associated with noninvasive BMIs, further research led to the development of semiinvasive BMIs. To improve the shortcoming of EEGs, electrocorticograms (ECoGs) were investigated. ECoG grids were placed in the subdural space in the hope that signal attenuation from the skull would be attenuated. It was found that a neuronal electrical signal was indeed markedly enhanced. Sadly, in these early studies, in spite of this greater data stream, patients were still unable to perform dexterous robot control.24 Nonetheless, ECoG devices produce signals with better resolution than do noninvasive BMIs, and they have a much lower risk of forming gliotic scar tissue than do fully invasive BMIs. In 2004, ECoG technology was first tried in adult human subjects by Leuthardt and Moran at Washington University in St. Louis. In 2006, a 14-year-old boy with epilepsy had an ECoG grid placed to localize his seizure focus. This ECoG grid was also linked to a computer running a BCI2000-based program that included the video game Space Invaders. The boy was then asked to perform various motor and speech tasks while the ECoG was acquired. The investigators were able to correlate the specific brain signals activated with certain movements and paired them with specific brain locations. The boy was then asked to play the simple two-dimensional Space Invaders game by moving his tongue and hand. The ECoG signals of his tongue and hand movements were correlated with the game cursor movement, which allowed him to play the game by simply moving his tongue and hand. His next task was to imagine the same movements, but not actually perform them with his hands or tongue. The result was the ability to play the video game by conscious thought. The results of this and similar studies along with advancements in fully invasive BMIs provide hope that conscious control of neuroprostheses can be achieved. Invasive BMI technology is the result of devices that are implanted directly into the gray matter of the brain. Deep brain stimulation is currently used in Parkinson disease, epilepsy, select pain conditions, and obsessive-compulsive disorder. Invasive devices produce the highest-quality signals of all BMI devices. The disadvantage is that they are prone to gliotic scar tissue formation, which can degrade the signals.35 Using this approach, Wessberg and his team were among the first group of researchers to demonstrate that primates could control robots in one- and three-dimensional (1-D and 3-D) motion with cortical signals from chronically implanted electrodes.36 In 1998, Kennedy and Bakay at Emory University implanted a cortical neuroprosthesis into Mr. Ray, a patient who was suffering from locked-in syndrome caused by a brain stem stroke in 1997. Using this neuroprosthesis, he acquired the ability to control a computer cursor and spell out words.37 In 2003, Donohue at Brown University and Cyberkinetics developed a 96-electrode array called BrainGate. In 2005, Mr. Nagle, who suffered from a C3 spinal cord injury, was implanted with BrainGate and was subsequently able to control a computer mouse such that he could make words, draw pictures, and change the channel on a television. He was also able to open and close a prosthetic hand. The patient was also able to control a multijointed robot performing basic motions using cortical signals.38 Currently, BrainGate2 is undergoing both safety and efficacy studies so as to obtain approval for a larger-cohort study with the intent of using the system to cortically control computers and other assistive devices. Although work continues to overcome the shortcoming of invasive BMIs’ limitations, most notably gliosis, targeted muscle reinnervation has emerged as a practical, though more limited, alternative. Targeted muscle reinnervation (TMR) was developed by Kuiken and colleagues at Northwestern University to enable greater control of upper-extremity prosthetic limbs and to restore some sensory feedback for arm/hand amputee patients.39–41 TMR is a method in which alternative muscle groups that are not biomechanically critical are deinnervated and then subsequently reinnervated with residual nerves from the amputated limb. This results in contractions in the targeted muscle responding to motor commands intended for the missing limb. EMG signals from these muscles are then used to actuate a prosthetic device. Traditionally, using myoelectric control, above-the-elbow amputees have to rely on body-powered technology, which uses bicycle cables to transfer the energy of chest and shoulder muscles to control a prosthetic limb. An improvement is using EMG signals from residual muscle groups to control the prosthesis. This strategy allows single-joint control and indirect control (e.g., residual biceps and triceps to control a prosthetic hook hand or wrist). TMR can overcome some of these limitations. TMR prosthetic control is intuitive to the patient because the EMG signal is generated by transferred residual limb nerves, unlike traditional myoelectric prosthetics, in which EMG signals have to be generated by muscles normally not involved in arm or wrist function. By transferring amputated upper-extremity nerves that had previously innervated the arm, to reinnervate nearby functionless muscle segments, the detection of the new EMG signals can be used to improve the control of a myoelectric prosthesis. For example, by transferring the median and distal radial nerve to other nerves innervating less functional muscle segments in the pectoralis region, the intact musculocutaneous (biceps) and proximal radial (triceps) signals are redirected. The EMG signals in the pectoralis region can then be captured to control elbow flexion and extension in a prosthetic device. The newly created EMG signals after successful multiple nerve transfers can also be used to control opening and closing of a prosthetic hand.26 Chronic implants in BMI devices typically fail over time because the neuronal signals are degraded by tissue immune response to these foreign bodies.35 TMR does not require implantation, so it does not have the issue of tissue foreign body response. With TMR, multiple yet independent EMG signals can be produced, leading to multiple simultaneous functions of the artificial limb.42 Pragmatically, this technology allows all existing commercially available myoelectric prostheses, such as powered wrists or elbows, to be used, so patients do not have to incur additional expense acquiring new protheses.40 Future research is being directed to provide improved thumb control as well as treatment for lower limb amputees. The hope is that nerves may be split further to provide even more independent signals, so that more functions can be controlled simultaneously with more degrees of freedom. One challenge for full-natural-control artificial limbs is that prosthetic devices do not provide the user with any direct sensory feedback. In targeted sensory reinnervation, the skin near or over the targeted muscle is denervated, then reinnervated with afferent fibers of the remaining hand nerves.39,43 This provides the amputee with a sense of the missing arm or hand being touched when the piece of skin that has been reinnervated is touched.44,45 Sensory feedback in this manner has not been achieved by any other forms of prostheses. With further advancements in neuroprostheses, the goal is to achieve motor and sensory function similar to its original state prior to its loss from disease or injury. There has been significant research and development with prosthetic devices to enhance sensory modalities. These implants are beyond the scope of a text focusing on spinal cord injury, but they illustrate the potential for more direct sensory feedback to the nerves and brain itself. They include cochlear implants, auditory brain stem implants capable of stimulating the cochlear nucleus or inferior colliculus, and visual implants in the retina, optic nerves, medial geniculate ganglia, or visual cortex. Research for the future is being directed in several exciting directions. As this field matures it is very possible that patients with spinal cord injury could use their neural signals to control an exoskeleton enabling ambulation, or paralyzed limbs could be replaced with prosthetic, neurally controlled limbs. Exciting areas outside of spinal injury include using word-specific neural signals that occur before the speech is vocalized to communicate without the use of vocalized speech, and the use of neuroprostheses to bridge lesions in the hippocampus to restore memory. Future-generation BMI electrode arrays are being designed with more biocompatible materials so as to evade the immunological response of the body in order for the implanted devices to function for decades.46,47 Along with this is the development of wireless versions of neuroprosthetic devices, making the devices more convenient for the user.48,49 The opinions expressed herein belong solely to the authors. They do not and should not be interpreted as belonging to or being endorsed by the Uniformed Services University of the Health Sciences, Defense Advanced Research Projects Agency, Walter Reed Army Medical Center, Department of the Army, or any branch of the US government.
Functional Restoration through Robotics
 Types of Neuroprostheses
Types of Neuroprostheses
Motor Prostheses
Functional Electrical Stimulation

Brain–Machine Interface
Noninvasive Brain–Machine Interfaces
Semiinvasive Brain–Machine Interfaces
Invasive Brain–Machine Interfaces
Targeted Muscle Reinnervation
Sensory Prosthetics
Targeted Reinnervation
Other Sensory Prosthetics
 Future Technologies
Future Technologies
Disclaimer
 Neuroprosthetic devices have dramatically helped improve the quality of life of disabled patients by reducing the physical and psychological impact of injury or disease.
Neuroprosthetic devices have dramatically helped improve the quality of life of disabled patients by reducing the physical and psychological impact of injury or disease.
 Functional restoration resulting from future advances in robotic prostheses combined with indirect and direct control mechanisms has the potential to outpace the functional expectations obtained with our standard biological repair techniques.
Functional restoration resulting from future advances in robotic prostheses combined with indirect and direct control mechanisms has the potential to outpace the functional expectations obtained with our standard biological repair techniques.
 Functional restoration through robotics has the potential to extend and enhance human capabilities.
Functional restoration through robotics has the potential to extend and enhance human capabilities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


