Free Vascularized Fibula Grafts
CASE PRESENTATION
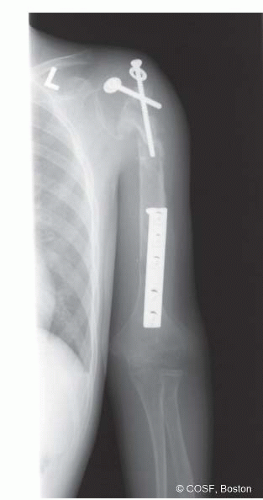
A 13-year-old male presents for consultation regarding a complicated fracture nonunion of the left humerus. Pertinent past medical history is significant for Ewing sarcoma of the humeral diaphysis, which had been previously treated with chemotherapy, tumor resection with intercalary allograft reconstruction, and radiation. He subsequently developed a fracture nonunion between the proximal host-graft junction (Figure 44-1). The patient has had persistent pain and functional limitations due to this fracture nonunion. He is disease free from the standpoint of his prior malignancy. He now presents for hand and upper extremity consultation in conjunction with orthopaedic oncology care.
CLINICAL QUESTIONS
What are the theoretical advantages of vascularized bone grafts?
What are the surgical indications for free vascularized fibula grafting (FVFG)?
How is the free vascularized fibula graft harvested?
What are the techniques of transferring the fibula with its physis and epiphysis to preserve growth potential?
What are the common complications of FVFG and strategies for their avoidance?
THE FUNDAMENTALS
Bone grafts are commonly used in all specialties of orthopaedic surgery. An understanding of the principles and applications of bone grafting is critical to the care of traumatic, developmental, and reconstructive musculoskeletal conditions, particularly in the pediatric patient. While most orthopaedic surgeons are familiar with the utilization of nonvascularized bone grafts and bone graft substitutes, the applications of vascularized bone grafts—and free vascular fibula grafts in particular—are often less well understood. The purpose of this chapter is to review the principles and techniques of FVFG, with particular attention to its applications in pediatric hand and upper limb surgery.
The Rationale for Vascularized Bone Grafts
Bone grafts and bone graft substitutes have a number of inherent properties that allow them to initiate, stimulate, and facilitate bony healing1,2 (Table 44.1). Osteoconduction refers to the process by which the graft provides a scaffold for the ingrowth of capillaries, perivascular tissue, and osteoprogenitor cells. Osteoinduction refers to the recruitment of osteoprogenitor cells from the surrounding tissue. Osteogenesis refers to the formation of new bone from either the host or graft tissue. In addition to these three properties, it is important to consider the mechanical strength and vascularity of the bone graft material.
Autogenous and allogeneic cortical and cancellous bone grafts are all, to varying degrees, osteoconductive, osteoinductive, and osteogenic. For these reasons, nonvascularized bone grafts are effective in facilitating bony healing. When appropriately utilized, nonvascularized bone grafts may be incorporated into the adjacent host bone through the process of “creeping substitution.” The bone graft material, through the invasion of capillaries, perivascular tissue, and inflammatory cells, is gradually revascularized and ultimately resorbed, allowing for the formation of new living bone, which is incorporated and remodeled into the host skeleton. However, this process takes time, during which the structural integrity and mechanical strength of the bone graft and host bone may be compromised.2
Vascularized bone grafts, by definition, retain their vascularity and thus are immediately viable. As a result, vascularized bone grafts obviate the need for incorporation by creeping substitution and may instead be incorporated into the adjacent host bone via primary (or secondary) bone healing. This process preserves the mechanical properties and structural integrity of the graft used, which confers greater strength and more immediate stability to the recipient site than nonvascularized grafts.
Free Vascularized Fibula Grafts
Wouldn’t it be interesting to have an occasional zebra in a horse race?
—The Vent
The fibula has long been recognized as an attractive choice for vascularized bone grafting procedures.3,4 The fibula bears only 15% of the axial load across the ankle, allowing for its use as an autogenous bone graft with minimal biomechanical consequences on the weight-bearing status of the lower limb.5 As the distal fibula also plays an important role in conferring rotational stability and restraint against lateral translation of the talus, the distal fibula is preserved during graft harvest to avoid subsequent ankle deformity or instability.6, 7, 8 and 9
Table 44.1 Properties of bone grafts | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Furthermore, the vascular supply to the fibula has been well established.4,9 The endosteal blood supply to the fibula is provided by a nutrient artery, which typically enters the posterior fibular cortex at the junction of the proximal one-third and distal two-thirds. This nutrient artery is a branch of the peroneal artery, which runs along the posterior aspect of the fibular diaphysis. The fibula receives additional vascularity via a number of segmental musculoperiosteal vessels, which also emanate from the peroneal artery. Based upon this understanding of the vascularity of the fibula, techniques of vascularized fibula graft harvest have been developed that preserve both the nutrient artery and the rich periosteal blood supply.
Free vascularized fibular grafting has a number of additional theoretical advantages over conventional, nonvascularized bone grafting techniques. Given the length of fibular diaphysis that may be harvested, free fibular grafts are well suited for the reconstruction of segmental defects of the long bones, providing both mechanical strength and biological stimulus for healing. Furthermore, based upon the fasciocutaneous arterial branches of the peroneal artery, skin, fascia, and muscle may be harvested concomitantly with the fibula to allow for more complex soft tissue reconstruction. Finally, given the ability to transfer the proximal fibular epiphysis with the diaphysis during FVFG, there is potential for preserving continued skeletal growth of the fibular graft.10
Despite its many theoretical advantages and applications, however, FVFG is technically challenging and confers its own set of inherent risks and potential complications. Sound microsurgical technique is essential in performing the required arterial and venous anastomoses and ensuring long-term graft viability. Furthermore, donor site morbidity has been well documented, and up to 10% of patients may subsequently develop ankle pain, instability, and/or progressive valgus deformity if fibula harvest is not performed with proper technique.7,8 Given these considerations, FVFG should be employed in specific clinical situations.
Table 44.2 Indications for FVFG | ||||
|---|---|---|---|---|
|
Currently, the indications for FVFG fall into two categories11 (Table 44.2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree