Chapter 11 Focal Atrial Tachycardia
Classification of Atrial Tachycardias
Therefore, ECG-based classifications are obsolete for this purpose, and a mechanistic classification of ATs has been proposed (Table 11-1).1,2 If the mechanism of AT or AFL can be elucidated, either through conventional mapping and entrainment or with special multipoint mapping techniques, description of the mechanism should be stated. However, even when using this classification, there are other tachycardias described in the literature that cannot be well classified because of inadequate understanding of their mechanisms, including “type II” AFL, inappropriate sinus tachycardia, and fibrillatory conduction from a focal source with rapid, regular discharge.
TABLE 11-1 Classification of Atrial Tachycardias
| Focal Atrial Tachycardia | ||
| Macroreentrant Atrial Tachycardia | Cavotricuspid isthmus-dependent atrial flutters | |
| Noncavotricuspid isthmus-dependent right atrial macroreentry (atypical atrial flutter) | ||
| Left atrial macroreentry (atypical atrial flutter) | ||
Pathophysiology
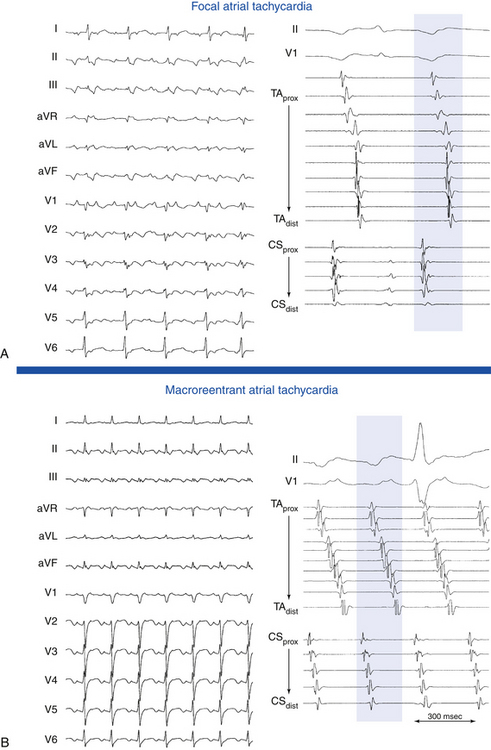
Focal AT is characterized by atrial activation starting rhythmically at a small area (focus), from which it spreads out centrifugally.1 “Focal” implies that the site of origin cannot be mapped spatially beyond a single point or a few adjacent points with the resolution of a standard 4-mm-tip catheter. In contrast, macroreentrant AT is defined by activation that can be recorded over the entire tachycardia CL around a large central obstacle, which is generally several centimeters in diameter.1 Relatively small reentry circuits may resemble focal AT, especially if limited numbers of endocardial recordings are collected. The term localized reentry has been used to refer to reentry in which the circuit is localized to a small area (covering a surface diameter of less than 3 cm) and does not have a central obstacle. Focal AT CL is usually longer than 250 milliseconds; however, it can be as short as 200 milliseconds. Over a prolonged period of observation (minutes to hours), the AT CL can exhibit significant variations.
Most focal ATs arise from the right atrium (RA), approximately two thirds of which are distributed along the long axis of the crista terminalis (cristal tachycardias) from the sinus node to the coronary sinus (CS) and atrioventricular (AV) junction (called the “ring of fire”), with an apparent gradation in frequency from superior to inferior.3,4 This particular anatomical distribution of ATs may be related to the marked anisotropy characterizing the region of the crista terminalis. Such anisotropy, which is related to the poor transverse cell-to-cell coupling, favors the development of microreentry by creating regions of slow conduction. Fractionated electrograms often seen at a successful AT ablation site may be markers of the requisite nonuniformly anisotropic substrate. In addition, the normal sinus pacemaker complex is distributed along the long axis of the crista terminalis. The presence of automatic tissue, together with relative cellular uncoupling, may be a requirement for abnormal automaticity such that a normal atrium is prevented from electrotonically inhibiting abnormal phase 4 depolarization. The presence of structural heart disease increases the probability of RA location of the AT, but from sites outside the crista terminalis.
The pulmonary vein (PV) ostia are the most common sites of origin of focal tachycardias within the left atrium (LA), and they account for between 3% and 29% of all focal ATs and approximately 67% of LA ATs.5–7 Other sites of AT clustering include the CS ostium (CS os),4,8–11 mitral and tricuspid annuli,3,5,12,13 bases of RA and LA appendages, para-Hisian region, and atrial septum. Experience gained during catheter ablation of atrial fibrillation (AF) has indicated that focal ATs also can arise within the vein of Marshall, superior vena cava (SVC),14 or inferior vena cava (IVC). ATs have been described originating from the noncoronary cusp of the aortic valve. The distribution of AT foci may differ, depending on the patient population.15,16
Available information suggests that focal activity can be caused by automaticity, triggered activity, or microreentry. There is some overlap based on the pharmacological characterization of these different AT mechanisms. Delineating the mechanism of focal AT, however, can be difficult, and the means of distinguishing focal AT mechanisms are fraught with exceptions.17 The major limiting factor in the analysis of mechanisms is the absence of a gold standard for determining the tachycardia mechanism, so these remain largely descriptive. In addition, there is a significant overlap in the EP characteristics of tachycardias with differing mechanisms.18 It is especially difficult to discriminate definitively between triggered activity and microreentry as a mechanism of focal AT; therefore, some investigators have classified focal ATs as automatic or nonautomatic.1 Furthermore, it is not clear that making such mechanistic distinctions among various types of focal ATs carries any clinical significance, although it is possible that such information could be useful in guiding drug therapy. In contrast, determination of the likely focal versus macroreentrant mechanism is critical for planning mapping and ablation strategy.
The term “incessant” is applied to an AT that is present for at least 50% of the time that a patient is monitored.19 Incessant AT frequently is automatic, but it can also be secondary to reentry or triggered activity. Incessant tachycardias occur in approximately 25% of patients with focal AT. Foci arising from the atrial appendages and PVs are frequently incessant.20
Arrhythmias originating from the PVs can have a critical role in the development of AF in susceptible individuals. Focal ATs arising from the PVs, however, appear to be a distinct clinical entity from the PV arrhythmias associated with AF. Patients with focal PV ATs do not seem to be at risk of AF in the long term; they resemble patients with focal ATs originating elsewhere with a localized isolated substrate that is successfully addressed with a focal ablative approach. Several potential explanations have been suggested for the different behavior. The underlying pathophysiological process in patients with AF is probably fundamentally different. It is a generalized process diffusely affecting the muscular sleeves in all four PVs, with frequently multiple PV foci originating distally (up to 2 to 4 cm) within the vein, compared with the focal nature of the process in patients with isolated PV tachycardia. Additionally, the CLs of the PV ATs are longer (mean CL 340 milliseconds) than the reported CLs of PV tachycardia in AF patients (130 milliseconds). The CL of AT also tends to be irregular in patients with AF but not in patients with PV AT. It is possible that foci with shorter CL and irregular activity may not conduct in a 1:1 fashion from the PV to the LA, with resulting fibrillatory conduction and AF. Furthermore, patients with AF tend to be older than those with PV AT and consequently more prone to more widespread atrial remodeling associated with age, hypertension, or other pathologic processes.6,7,21,22
Up to 10% of patients with focal AT have ATs with more than one focus. In these patients, the AT appears to have different EP characteristics from focal AT with a single focus, because it tends to involve the LA and has greater cardiovascular comorbidity, shorter CL, longer total activation time, and lower acute and long-term success rates of catheter ablation.23
Other forms of AT in which EP testing and catheter ablation are not indicated are beyond the scope of this chapter and are discussed briefly. Multifocal AT is usually caused by enhanced automaticity and is characterized by varying morphology of the P waves and the PR interval (Fig. 11-1). This finding suggests that the pacemaker arises in different atrial locations, but a single focus with different exit pathways or abnormalities in intraatrial conduction can produce identical electrocardiographic manifestations. Nonparoxysmal AT typically occurs in patients with significant heart disease or digitalis toxicity, or both. The latter may be caused by triggered activity. Repetitive automatic AT, also called repetitive focal AT, is thought to be caused in most cases by enhanced automaticity. It frequently occurs in patients with structural heart disease and is often related to some acute event, such as a myocardial infarction, pulmonary decompensation, infection, alcohol excess, hypokalemia, hypoxia, or use of stimulants, cocaine, or theophylline.
Early studies of sinus node function described the response to a single atrial extrastimulus (AES). The curve produced by plotting return cycles against the coupling interval could be divided into several phases, including compensatory pause, reset, interpolation, and reentry. Sinus node reentrant tachycardia was described on the basis of these findings as a tachycardia that could be induced and terminated by programmed electrical stimulation with a P wave morphology identical or similar to sinus P waves and tachycardia CLs of 350 to 550 milliseconds (see Fig. 16-3).24 There have been some reports of endocardial ablation of sinus node reentrant tachycardias identified by these criteria. However, the precise identification of sinus node reentrant tachycardia remains elusive. The mechanism of focal AT can be reentrant, and the CL of such ATs completely overlaps that described for sinus node reentrant tachycardia. The location of AT foci is often along the crista terminalis, very close to the supposed location of the sinus node. An added problem is that the origin of the sinus activation can be variable, according to epicardial mapping studies. Furthermore, endocardial origin of sinus activation during normal sinus rhythm (NSR) has not been systematically studied in humans, and specific criteria do not exist to pinpoint a specific sinus node area. Finally, reentry strictly limited to the sinus node area has never been demonstrated and has even been questioned.24
Clinical Considerations
Epidemiology
Nonsustained AT is frequently found on Holter recordings and is seldom associated with symptoms. Sustained focal ATs are relatively infrequent; they are diagnosed in approximately 5% to 15% of patients referred for catheter ablation of supraventricular tachycardia (SVT).25 However, ATs comprise a progressively greater proportion of paroxysmal SVTs with increasing age and account for 23% in patients older than 70 years. Age-related changes in the atrial EP substrate, including cellular coupling and autonomic influences, can contribute to the increased incidence of AT in older individuals.25 In adults, focal AT can occur in the absence of cardiac disease, but it is often associated with underlying cardiac abnormalities. Not infrequently, two or more foci of AT can be found in the same patient.26
Clinical Presentation
Focal ATs can manifest as paroxysmal or incessant tachycardias. When paroxysmal, AT manifests with the clinical syndrome of paroxysmal SVT (discussed in Chap. 20). Symptoms, however, may be more severe or associated with cardiac decompensation caused by the higher prevalence of structural heart disease in this group of patients. Incessant tachycardia can result in tachycardia-induced cardiomyopathy and manifest with symptoms of congestive heart failure. AT can also manifest as a frequently repetitive tachycardia, with frequent episodes of AT interrupted by short periods of NSR. The repetitive type may be tolerated well for years. It may cause symptoms only in cases of fast heart rates during phases of tachycardia, and it infrequently induces dilated cardiomyopathy.
In one study, tachycardia-induced ventricular cardiomyopathy occurred in 10% of the total population of patients with focal AT. Incessant tachycardia was necessary for the development of cardiomyopathy, and cardiomyopathy was observed in approximately one third of patients with incessant AT. Incessant tachycardias precipitating cardiomyopathy characteristically tend to have slower atrial rates as compared with ATs not associated with cardiomyopathy. In contrast to patients with rapid paroxysmal AT, incessant tachycardia may produce negligible symptoms and may go unrecognized by the patient until symptoms related to cardiomyopathy develop.20
Principles of Management
Short-Term Management
The usual acute therapy for AT consists of intravenous beta blockers or calcium channel blockers either to terminate the arrhythmia, which is rare, or to achieve rate control through AV block, which is often difficult.19 Direct suppression of the tachycardia focus may be achieved by the use of intravenous class IA and IC or class III agents. Intravenous class IA or IC agents may be taken by patients without heart failure, whereas intravenous amiodarone is preferred for those with poor LV function.19
Long-Term Management
As with other forms of SVT, AT is usually benign and associated with only mild to moderate symptoms. Thus, most patients with AT are treated initially with medical therapy. Patient preference and refractoriness to pharmacological therapy are reasonable indications for catheter ablation.19
The efficacy of antiarrhythmic drugs is poorly defined because the clinical definition of focal ATs is often not rigorous. No large studies have been conducted to assess the effect of pharmacological treatment in patients with focal ATs, but both paroxysmal and, especially, incessant ATs are reported to be difficult to treat medically. Available data support a recommendation for initial therapy with calcium channel blockers or beta blockers because these agents may prove to be effective and have minimal side effects. If these drugs are unsuccessful, then class IA agents, class IC (flecainide and propafenone) agents in combination with an AV node (AVN) blocking agent, or class III agents (sotalol and amiodarone) may be tried; however, the potential benefit should be balanced by the potential risks of proarrhythmia and toxicity. Because ATs often occur in older patients and in the context of structural heart disease, class IC agents should be used only after cardiomyopathy and coronary artery disease are excluded.19
For patients with drug-refractory AT or incessant AT, especially when tachycardia-induced cardiomyopathy has developed, the best therapy appears to be catheter ablation of the AT focus. In case reports and small series, patients with tachycardia-induced cardiomyopathy frequently have complete resolution of LV dysfunction with successful RF catheter ablation of the atrial focus.19,20 Regardless of whether the arrhythmia is caused by abnormal automaticity, triggered activity, or microreentry, focal AT is ablated by targeting the site of origin of the AT. Catheter ablation for focal AT carries a success rate of more than 90%, with a recurrence rate of 9%. The incidence of significant complications is relatively low (1% to 3%) in experienced centers.
Electrocardiographic Features
P Wave Morphology
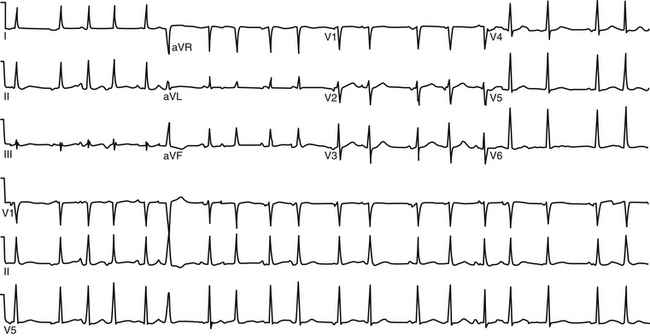
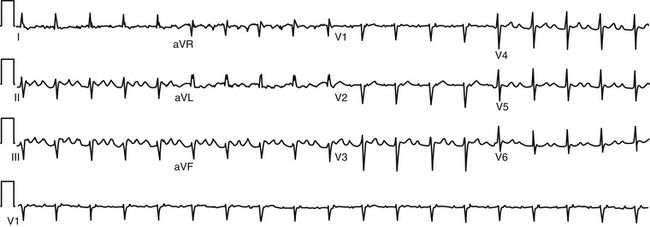
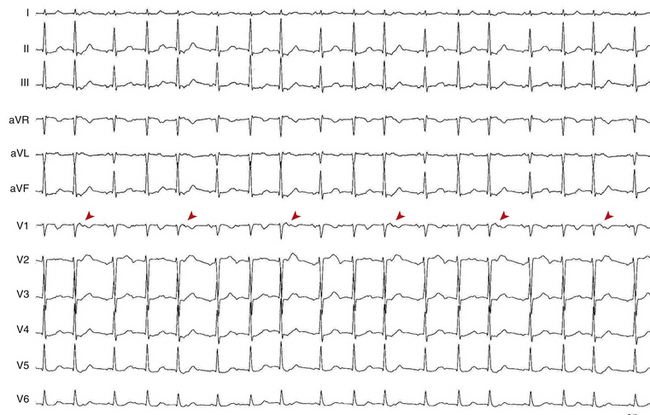
During AT, typically there are discrete P waves at rates of 130 to 240 beats/min, but possibly as slow as 100 beats/min or as fast as 300 beats/min. Antiarrhythmic drugs can slow the tachycardia rate without abolishing the AT. Classically, there are clearly defined isoelectric intervals between the P waves in all leads (Fig. 11-2). However, in the presence of rapid rates, intraatrial conduction disturbances, or both, the P waves can be broad, and there may be no isoelectric baseline. In these cases, the ECG shows an AFL pattern (continuous undulation without isoelectric baseline; Fig. 11-3).1 Nevertheless, one report demonstrated a high sensitivity (90%) and specificity (90%) of quantitative ECG indexes of shorter atrial activation and longer diastolic intervals in distinguishing focal from macroreentrant AT. This approach was effective in cases with and without 1:1 AV conduction and when the P or flutter waves overlay T waves.27
P wave morphology depends on the location of the atrial focus, and it can be used to localize the site of origin of the AT approximately. However, the P wave can be partially masked by the preceding ST segment or T wave, or both. Vagal maneuvers and adenosine infusion to provide transient AV block can be used to obtain a clear view of the P wave, assuming that the tachycardia does not terminate with AV block. The compensatory pause following a premature ventricular complex during the AT can also be used to delineate P wave morphology (Fig. 11-4). It has been suggested that the multilead body surface potential recording can be used to help localize the site of origin of the tachycardia.
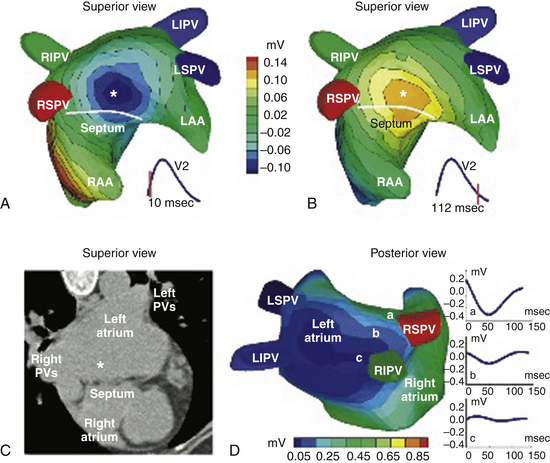
One report described a clinical application of ECG imaging (ECGI) as an adjunctive noninvasive technology to identify the site of origin of a focal AT accurately prior to catheter ablation (Fig. 11-5).28
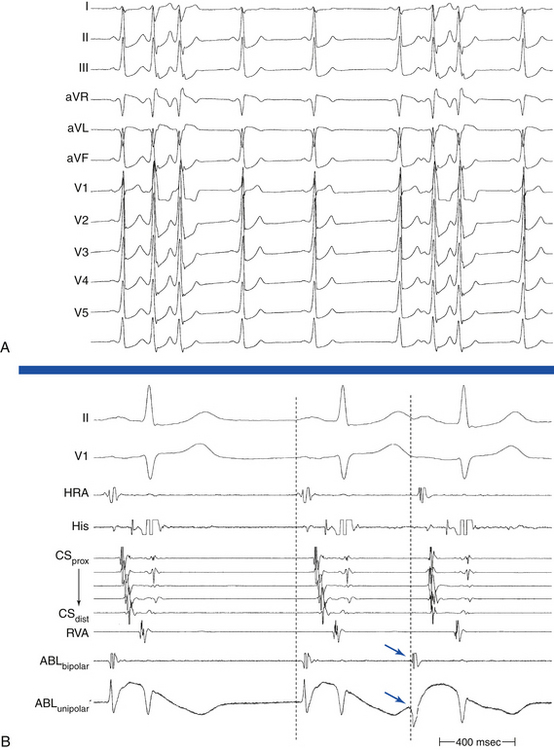
Focal automatic ATs start with a P wave identical to the P wave during the arrhythmia, and the rate generally increases gradually (warms up) over the first few seconds. In comparison, intraatrial reentry or triggered-activity AT is usually initiated by a P wave from a premature atrial complex (PAC) that generally differs in morphology from the P wave during the established arrhythmia (Fig. 11-6).
P/QRS Relationship
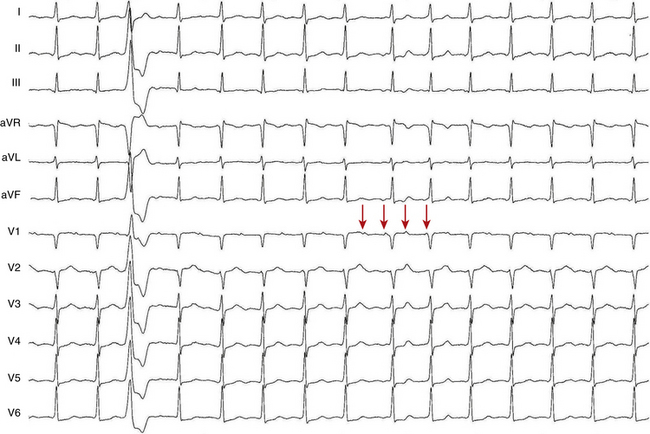
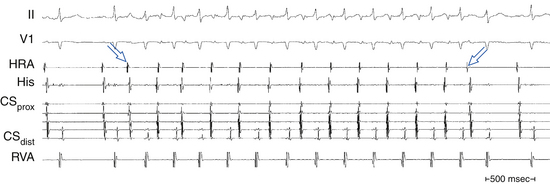
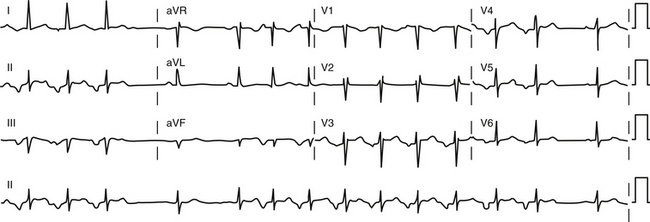
The atrial to ventricular relationship is usually 1:1 during ATs, but Wenckebach (Fig. 11-7) or 2:1 AV block (see Figs. 11-3 and 11-4) can occur at rapid rates, in the presence of AVN disease, or in the presence of drugs that slow AVN conduction. The presence of AV block during an SVT strongly suggests AT, excludes AV reentrant tachycardia (AVRT), and renders AVN reentrant tachycardia (AVNRT) unlikely.
Localization of the Atrial Tachycardia Site of Origin Using P Wave Morphology
General Observations Relating P Wave Morphology to Site of Origin of Atrial Tachycardia
P wave morphology provides a useful guide to the localization of focal AT in patients without structural heart disease. In patients with prior surgery or extensive atrial ablation or in those with significant structural heart disease, activation patterns can be altered, significantly rendering P wave morphology less helpful.29
ECG lead V1 is the most useful in identifying the likely anatomical site of origin for focal AT. Lead V1 is located to the right and anteriorly in relation to the atria, which should be considered anatomically as right anterior and left posterior structures. Thus, for example, tachycardias originating from the tricuspid annulus have negative P waves in lead V1 because of the anterior and rightward location of this structure (i.e., activation travels away from lead V1). The P wave in lead V1 is universally positive for tachycardias originating from the PVs because of the posterior location of these structures (i.e., the impulse travels toward lead V1).6,30,31
Several features of P wave morphology can help predict whether an AT is arising from the RA or the LA. In one report, a negative or biphasic (positive-negative) P wave in lead V1 was associated with 100% specificity, 100% positive predictive value, 69% sensitivity, and 66% negative predictive value for a tachycardia arising from the RA. Conversely, a positive or negative-positive biphasic P wave in lead V1 had 100% sensitivity, 81% specificity, 76% positive predictive value, and 100% negative predictive value for an LA origin. A positive or biphasic P wave in lead aVL predicted an RA focus; however, some patients with right superior PV foci had positive P waves in lead aVL. An isoelectric or negative P wave in lead I was 100% specific (but only 50% sensitive) for LA foci.6
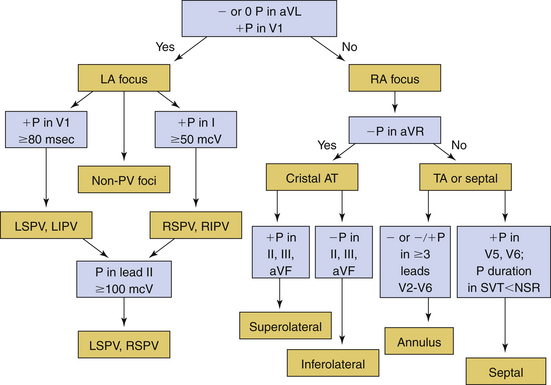
Several algorithms have been proposed for ECG localization of focal AT using P wave morphology. Figure 11-8 summarizes some of those algorithms.6
Right Atrial Tachycardias
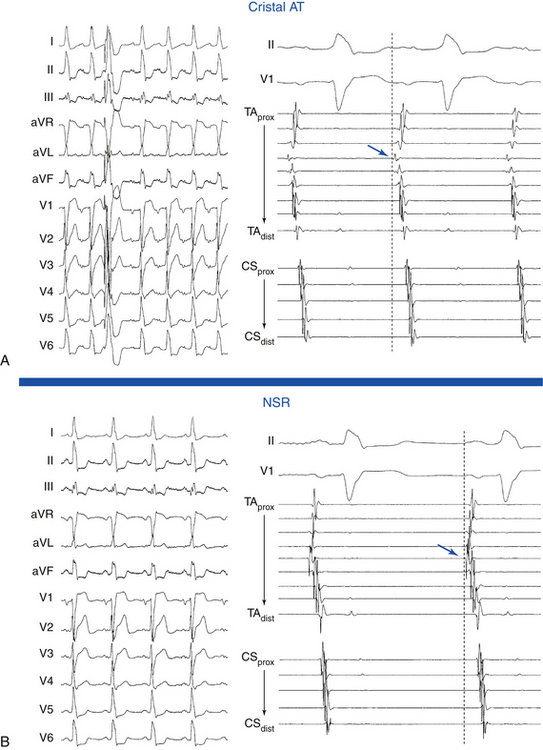
Cristal ATs (i.e., ATs arising from the crista terminalis) are characterized by right-to-left activation sequence resulting in P waves that are positive and broad in leads I and II, positive in lead aVL, and biphasic in lead V1 (Fig. 11-9). A negative P wave in lead aVR predicts a cristal origin compared with anterior RA foci with a sensitivity of 100% and specificity of 93%. Biphasic P waves (positive-negative) in lead V1 (or positive V1 during both tachycardia and sinus rhythm), positive P waves in leads I and II, and negative P waves in lead aVR predict a cristal origin with 93% sensitivity, 95% specificity, 84% positive predictive value, and 98% negative predictive value. Although there can be overlap in P wave morphology between the superior crista and the right superior PV as a result of the anatomical proximity of the two sites, these can be distinguishable on the basis of changes to the P wave morphology in lead V1 during tachycardia as compared with sinus P waves. In right superior PV AT, P waves in lead V1 are invariably upright during tachycardia and biphasic (positive-negative) in sinus rhythm. When cristal AT has an upright P wave in lead V1 (approximately 10%), it is invariably also upright during sinus rhythm. High, middle, or low cristal locations can be identified by P wave polarity in the inferior leads.29
In the setting of posteroseptal ATs (originating below and around the CS os), the P wave is positive in lead V1, negative in the inferior leads, and positive in leads aVL and aVR. Those ATs can mimic fast-slow AVNRT or orthodromic AVRT using a posteroseptal BT.
The circumference of the tricuspid annulus is the second most common site of origin for RA tachycardias. A common feature of tricuspid annular ATs is the presence of an inverted P wave in leads V1 and V2 with late precordial transition to an upright appearance. The nonseptal sites demonstrate negative P waves in lead V1, whereas anteroinferior sites tend to have inverted P waves across the precordial leads, and superior sites closer to the septum show transition from negative in lead V1 through biphasic to upright in the lateral precordial leads.3 In general, the polarity of leads II and III is deeply negative for an inferoanterior location and low amplitude, positive, or biphasic for a superior location. Additionally, tricuspid annular ATs typically have positive P waves in lead aVL and positive or isoelectric P waves in lead I.29
Focal ATs originating from the RA appendage typically originate from the lateral base of the appendage but are also well described from an apical location. Because of their close anatomical proximity, these tachycardias are generally indistinguishable from superior tricuspid annular foci; they exhibit negative P waves in leads V1 and V2 and variable precordial transition to positive in lead V6. P waves in the inferior leads characteristically have low positive amplitudes.29,32
Left Atrial Tachycardias
ATs arising from the PVs are characterized by entirely positive P waves in lead V1 (in 100% of cases) and across the precordial leads, isoelectric or negative waves in lead aVL in 86%, and negative waves in lead aVR in 96%. Lead aVL can be biphasic or positive in right-sided PV ATs.7 Compared with the right-sided PVs, the left-sided PV foci have several characteristics: positive notching in the P waves in two or more surface leads, an isoelectric or negative P wave in lead I, P wave amplitude in lead III/II ratio greater than 0.8, and broad P waves in lead V1 (Fig. 11-10). Right-sided PV foci usually have positive P waves in lead I. ATs arising from the superior PVs invariably have a positive P wave in the inferior leads. In contrast, ATs arising from the inferior PVs can have inverted, low-amplitude positive or isoelectric inferior P waves. However, because of the close proximity of the superior and inferior veins and marked anatomical variation, P wave morphology generally is of greater accuracy in distinguishing right-sided from left-sided PVs, in contrast to distinguishing superior from inferior PVs.
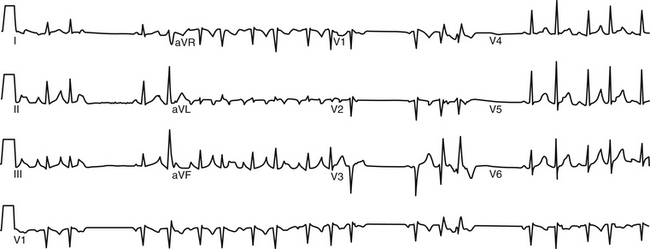
FIGURE 11-10 Surface ECG of nonsustained focal atrial tachycardia originating from the ostium of the left superior pulmonary vein.
ATs arising from the right superior PV are associated with P waves that are narrow and positive in the inferior leads, of equal amplitude in leads II and III, positive in lead V1, and isoelectric in lead I. The right superior PV is a common site of origin for LA ATs. It is only a few centimeters from the sinus node. Activation rapidly crosses the septum via Bachmann’s bundle to activate the RA in a fashion similar to NSR, a feature explaining the similarities in P wave morphology. However, whereas the P wave is biphasic in lead V1 during NSR, it is positive in that lead during right superior PV AT (Fig. 11-11).7
Despite prior posterior LA ablation, the surface ECG morphology of ATs originating from the PV ostia in patients with prior AF ablation procedures is similar to those in patients without prior ablation. However, ATs originating from the bottom of the right or left PVs after prior PV isolation can have a significant negative component or can be completely negative in the inferior leads. This may be related to prior ablation in the superoposterior LA or to a more inferior origin of the tachycardia after prior ablation outside the PV ostium.33
In the setting of LA appendage ATs, the P wave is positive in the inferior leads (more positive in lead III than in II), positive in lead V1, and negative in leads I and aVL. The LA appendage is closely approximated to the left superior PV, and, as such, ATs arising from those locations tend to have similar P wave morphologies. When P wave morphology suggestive of a left superior PV focus (broad upright and notched in lead V1 and in the inferior leads) exhibits a deeply inverted P wave in lead I, an origin from the LA appendage should be strongly suspected. P waves are typically more positive in lead III than in lead II. A negative P wave in leads I and aVL predicts an LA appendage focus with a sensitivity and specificity of 92% and 97%, respectively.29
Mitral annular ATs typically cluster at the superior aspect of the mitral annulus in close proximity to the aortomitral continuity. ATs originating in this circumscribed area are characterized by P waves with an initial narrow negative deflection in lead V1 followed by a positive deflection. The positivity of the P wave becomes progressively less from V1 through V6. The P wave is negative in leads I and aVL and isoelectric or slightly positive in the inferior leads.34
ATs arising from the CS musculature typically demonstrate deeply inverted P waves in the inferior leads. Lead V1 usually exhibits isoelectric-positive or biphasic (negative-positive) P waves, with variable precordial transition. P waves are invariably positive in leads aVL and aVR.4 Compared with AT originating from the CS os, AT originating from 3 to 4 cm into the body of the CS have broad and upright P waves in lead V1.29
ATs have been described originating from the noncoronary cusp of the aortic valve. Because of the close anatomical proximity, the P wave morphology is similar to that of ATs arising from the aortomitral continuity. The P waves in leads V1 and V2 are also characteristically negative. However, unlike aortomitral ATs, those arising from the noncoronary cusp demonstrate upright P waves in leads I and aVL. In the inferior leads, the P waves are biphasic negative-positive but of low amplitude.15,16,29
Electrophysiological Testing
Induction of Tachycardia
Frequently, AT foci can become inactive in the EP laboratory environment because of sedative medications, changes in autonomic tone that can be caused by prolonged supine position, patient anxiety, deviation from normal diet, or other changes in daily activities that can have an impact on the circadian variation of AT activity. Thus, in preparation for an AT ablation procedure, several measures should be undertaken. Antiarrhythmic drugs should be discontinued for at least five half-lives before the EP study. Sedation should be minimized throughout the procedure. It may be an appropriate policy to monitor the patient in the EP laboratory initially without sedation. If no spontaneous tachycardia is induced, isoproterenol is administered. If no AT can be induced, a single quadripolar catheter is placed in the RA, and programmed electrical stimulation is performed without and, if no AT is induced, with isoproterenol infusion. If AT remains quiescent, the procedure is aborted and retried at a future date. If AT is inducible at any step, the full EP catheter arrangement and EP study are undertaken (Table 11-2).17,24,31
TABLE 11-2 Programmed Electrical Stimulation Protocol for Electrophysiological Testing of Atrial Tachycardia
AES = atrial extrastimulation; CL = cycle length; CS = coronary sinus; ERP = effective refractory period; RA = right atrium; RV = right ventricle; VA = ventricular-atrial; VES = ventricular extrastimulation.
Initiation by Atrial Extrastimulation or Atrial Pacing
In the setting of microreentry and triggered activity, the first tachycardia P wave is different from subsequent P waves; the first P wave is usually a PAC or an AES that is necessary to start the AT (see Fig. 11-6). In contrast, the first tachycardia P wave and subsequent P waves during automatic AT are identical; the AT does not require a PAC to start (Fig. 11-12). Furthermore, the automatic AT CL tends to shorten progressively (warm up) for several beats until its ultimate rate is achieved.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree