Wayne G. Paprosky
Femoral Bone Loss Classification
BACKGROUND
Primary total hip arthroplasty (THA) is one of the most effective procedures in medicine for the treatment of end-stage hip arthritis based on its ability to control pain and restore function. As the number of primary THA procedures performed continues to rise, the burden of revision THA procedures is also expected to increase. Implant longevity continues to be of utmost importance. Modes of failure necessitating total hip revision include aseptic loosening, infection, instability, polyethylene wear, osteolysis, periprosthetic fracture, component malposition, and catastrophic implant failure. Loss of femoral bone stock may be associated with each of these and may be exacerbated by iatrogenic bone loss during implant removal.
Proper preoperative patient assessment and preoperative planning is essential when evaluating and treating femoral bone loss. The remaining bone stock dictates the reconstructive options available and the type of stem that should be utilized for optimal fixation. This chapter provides an approach to evaluating and treating femoral bone loss in revision THA. The clinical results of several reconstruction techniques are presented and should be taken into account when choosing a suitable treatment option.
PREOPERATIVE PATIENT EVALUATION
Thorough preoperative patient assessment is critical when preparing for femoral component revision. All prior procedures and a detailed history of all perioperative complications should be obtained. The patient history must document the location, type character, duration, temporal nature, exacerbating and remitting factors (i.e., activity-related symptoms), and previous treatments.
The lack of a pain-free interval following primary THA prompts questioning of the indication for surgery or may indicate low-grade sepsis. A diagnosis of deep infection must be ruled out prior to undertaking revision surgery. Serum sedimentation rate (ESR) and C-reactive protein must be obtained in all revision patients. Elevated serum inflammatory markers should prompt a preoperative hip aspiration. Synovial fluid analysis includes anaerobic and aerobic cultures as well as a cell count. A WBC count of 1,000 to 3,000 and a differential >60% segmented neutrophils is suspicious for infection.1,2
Physical examination includes an assessment of the patient’s general health, the lumbosacral spine, contralateral limb, and detailed examination of the affected hip and lower extremity. A detailed motor, sensory, and vascular examination must also be performed. The patient’s gait should be evaluated for the presence of painful ambulation (antalgic gait) and presence of compensatory gait patterns (i.e., Trendelenburg gait). Leg-length discrepancy should also be assessed.
PREOPERATIVE RADIOGRAPHIC EVALUATION
Preoperative radiographic assessment includes projections that visualize the entire prosthesis. An anteroposterior (AP) pelvis and a lateral of the hip usually suffice, but an AP of the hip may be necessary. An adequate AP pelvis x-ray allows for assessment of leg length. Evaluation of the acetabulum is also important because it may influence the type of femoral reconstruction chosen.
Plain radiographs should be assessed for component position, component loosening, location and degree of osteolysis, and quality and quantity of remaining bone stock. Radiographs should be evaluated for subtle interface changes suggesting loosening. Serial radiographs are important to document interval changes in prosthetic position.
When considering a loose cementless femoral device, components can be categorized as ingrown, stable fibrous, or unstable. Ingrown femoral components typically have “spot welds” of endosteal bone at the bone-implant interface. Stable fibrous components may show nonprogressive reactive lines between the porous coating and endosteum. A small pedestal may be present, but component subsidence is usually absent. Unstable components reveal progressive, nonlinear radiolucencies at the bone-implant interface, component migration, and a large supportive distal pedestal. Bead shedding may also be present.3
In the setting of a loose femoral component, more commonly a loose cemented stem, the proximal femur often remodels into varus and retroversion (proximal femoral remodeling) (Fig. 20-1). Recognizing such remodeling preoperatively minimizes the risk for cortical perforation, intraoperative fracture, and undersizing of the implant. An extended trochanteric osteotomy (ETO) is often required in the presence of such remodeling.4

FIGURE 20-1. Intraoperative image of a proximal femur encountered during surgical treatment of a periprosthetic femur fracture. Note the proximal femoral varus remodeling.
Bone loss associated with osteolysis on plain radiographs is often less severe than that encountered at the time of surgery. Computed tomography can be used as an adjunct for further defining osteolysis and severity of femoral bone loss.5
PREOPERATIVE PLANNING
Surgical Approach Alternatives
The surgical approach for revision surgery is based on surgeon preference, comfort, and utility for the planned reconstruction. The need for additional exposure (i.e., osteotomy), the degree and location of bone deficits, and the presence of distorted anatomy (e.g., heterotopic ossification), in conjunction with patient factors (e.g., high risk for instability), also influence the choice of surgical approach. The posterolateral approach affords excellent acetabular and femoral exposure but is associated with a higher risk for postoperative instability.
A series of femoral osteotomies are available for enhanced exposure. Osteotomies about the greater trochanter can be categorized as standard singleplane osteotomy, trochanteric slide, Wagner osteotomy, and ETO (Fig. 20-2). Fixation of these osteotomies includes the use of various wire constructs, cables, cable or claw plates, or a combination.6
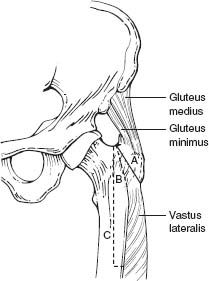
FIGURE 20-2. Depiction of different osteotomies around the greater trochanter and proximal femur. (Adapted from Archibeck MJ, et al. Trochanteric osteotomy and fixation during total hip arthroplasty. J Am Acad Orthop Surg 2003;11(3):163–173.)
ETO is most commonly used in the setting of revision THA for adjunctive acetabular exposure and femoral component removal. In 1995, the Wagner osteotomy (anterior trochanteric osteotomy) was modified to include an extended lateral trochanteric osteotomy, reflecting the abductor complex in continuity with the lateral one third of the proximal femur.6,7
The length of the ETO is planned preoperatively, allowing for at least 4 to 6 cm of diaphyseal stem engagement. The osteotomy is typically performed through a posterolateral approach. The linea aspera is exposed, and the length of the planned osteotomy is measured from the tip of the greater trochanter. The osteotomy is started along the posterior margin of the femur with a microsagittal saw or pencil tip burr. The transverse component at the distal extent is made with rounded corners and extends anteriorly to cover one third of the femoral shaft circumference. The anterior limb of the osteotomy is initiated, and a series of broad osteotomes are introduced from posterior to anterior and lifted simultaneously to open the osteotomy. The osteotomy fragment, which includes the entire greater trochanter and the lateral one third of the femoral cortex, is retracted anteriorly to maximize exposure. A prophylactic cerclage wire is placed distally to prevent crack propagation.6,8,9
Femoral Component Extraction Techniques
Preoperative planning requires identification of current implants that are in place; operative reports for prior procedures are also helpful. Although not the focus of this chapter, detailed evaluation of the acetabular component is also required. The following is a list of issues to consider when revising a femoral stem: (a) implant manufacturer, (b) type of component (monoblock vs. modular), (c) presence of modularity (head vs. neck), (d) design specifics (i.e., anteversion built into the stem), (e) type of femoral neck taper, (f) head and neck options available, and (g) the track record of the stem in place.
If femoral component revision is required based on preoperative planning, component removal with the least amount of additional bone loss is paramount. Having certain instruments available can minimize difficulty in removing the stem. These instruments include (a) manufacturespecific explant tools (when available), (b) flexible osteotomes, (c) trephines, (d) high-speed burrs, (e) ultrasonic cement removal instruments, (f) and universal extraction tools. The decision to remove a well-fixed implant must be made carefully.10
It is important to recognize factors that may make component extraction difficult. These include well-fixed cemented implants, extensively porous-coated implants, proximally coated implants with grit blasting distally, proximal femoral remodeling, and trochanteric overgrowth. Explantation can be facilitated with the use of adjunctive exposure (described above). Additional techniques include the use of controlled cortical perforations or cortical windowing.
The ETO is most commonly used for implant removal and femoral reconstruction in the setting of proximal femoral remodeling. The results of ETO use in femoral revision THA have been favorable. Paprosky and Sporer evaluated 122 revision THAs performed with the use of an ETO.7 They reported a 98% (120/122) union rate of the osteotomized fragment, with a mean follow-up time of 2.6 years. There were four fractures of the osteotomized fragment and only one case in which the fragment migrated >2 mm proximally. All revisions were performed with extensively porous-coated stems. About 112 of 122 hips (92%) attained stable bony ingrowth, 9 of 112 hips (7%) attained stable fibrous ingrowth, and only 1 hip required re-revision for instability. Other authors have reported similarly predictable results with healing rates of the osteotomy fragment between 96% and 98%.11–13
Complete circumferential exposure of the proximal portion of the femoral component is important for safe component removal. A high-speed pencil tip burr in conjunction with a series of flexible osteotomes is used to remove all fibrous tissue and trochanteric overgrowth surrounding the “shoulder” of the femoral component. Implants that are fibrous stable still require complete clearance of the “shoulder” prior to removal. Do not force a space-occupying instrument (e.g., osteotome) between the prosthesis and the bone due to a heightened risk for periprosthetic femoral fracture, specifically the greater trochanter. An implantspecific or universal extractor can be attached to the neck or trunnion of the stem, and extraction can be attempted by applying an axial force in line with the long axis of the component.
The above-described technique is often successful for the removal of well-fixed cementless proximally coated or loose extensively porous-coated implants. This technique is often not adequate for removal of proximally coated implants that have undergone bone ongrowth to the grit-blasted distal portion of the implant or for removal of well-fixed extensively porous-coated devices. An ETO is often used for safe removal of such implants.
Following the osteotomy, an extensively porous-coated stem is cut with a metal-cutting burr just distal to the flare of the metaphyseal portion. A series of trephines on power are used to remove the remaining cylindrical portion of the stem. This process is often time consuming and usually requires several trephines. An intraoperative x-ray can ensure the trephine is collinear with the remaining stem. This technique is also used for removal of a fractured extensively porous-coated stem.
In general, cemented stems are of two varieties: smooth and polished or matte finished. Polished cemented stems typically are removed without difficulty from the cement mantle. Well-fixed matte finished stems are difficult to remove and may require an ETO. An ETO may still be used with loose stems to facilitate removal of the remaining cement. An ultrasound-guided cement removal tool may also be required for complete cement removal.
An ETO is very versatile in its use and should be a part of the armamentarium in the treatment of periprosthetic fractures and periprosthetic infection. Levine et al.14,15 demonstrated 95% healing of an ETO when utilized in the setting of periprosthetic fracture and periprosthetic infection for femoral component removal.
FEMORAL BONE LOSS CLASSIFICATIONS
The American Academy of Orthopaedic Surgeons introduced a femoral bone loss classification based on the presence of segmental, cavitary, or combined defects16,17 (Table 20.1). This classification is simple in its organization; however, it is not quantitative and the practical application is limited. A series of other classifications have been described, but none have been as popular as the Paprosky classification for femoral bone loss.17,19,20 This classification is comprehensive and provides treatment recommendations based on the location and degree of bone loss.17,19
TABLE 20.1 American Academy of Orthopaedic Surgeons Femoral Bone Loss Classification
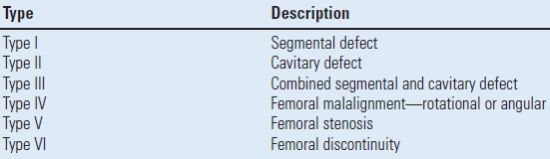
Ref.18 D’Antonio JA, et al. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res 1989;243:126–137.
The Paprosky femoral bone loss classification is based on three variables: (a) the location of bone loss (metaphyseal vs. diaphyseal), (b) the degree of remaining support of the proximal femur (i.e., degree of cancellous bone loss), and (c) the amount of isthmus remaining for diaphyseal fixation (Table 20.2). These three variables allow for objective assessment of bone loss and provide reconstructive options based on the pattern of femoral bone loss.
TABLE 20.2 Paprosky Femoral Bone Loss Classification
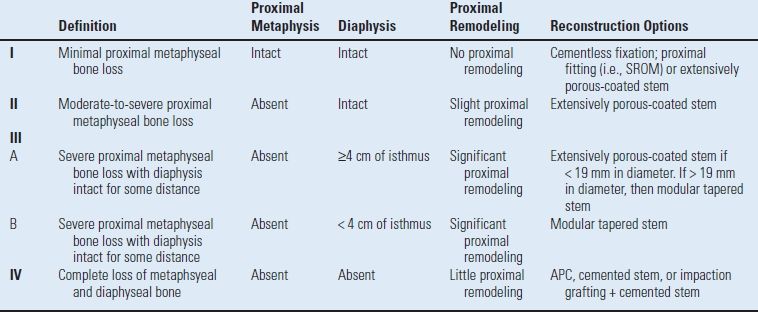
Paprosky W, Aribindi R. Hip replacement: treatment of femoral bone loss using distal bypass fixation. Instr Course Lect 2000;49:119–130; Pak JH, et al. Femoral strut allografts in cementless revision total hip arthroplasty. Clin Orthop Relat Res 1993;295:172–178.
Type I femoral bone loss maintains the proximal metaphyseal bone and typically does not exhibit proximal femoral remodeling. Type I defects can be treated with a cementless, proximally porous-coated implant or with a standard-length extensively porous-coated or other standard-length distally fixed implants17,19 (Figs. 20-3 and 20-4).

FIGURE 20-3. Illustration of Paprosky type I femoral defect. (Radiograph from Della Valle CJ, Paprosky WG. The femur in revision total hip arthroplasty evaluation and classification. Clin Orthop Relat Res 2004;(420):55–62.)
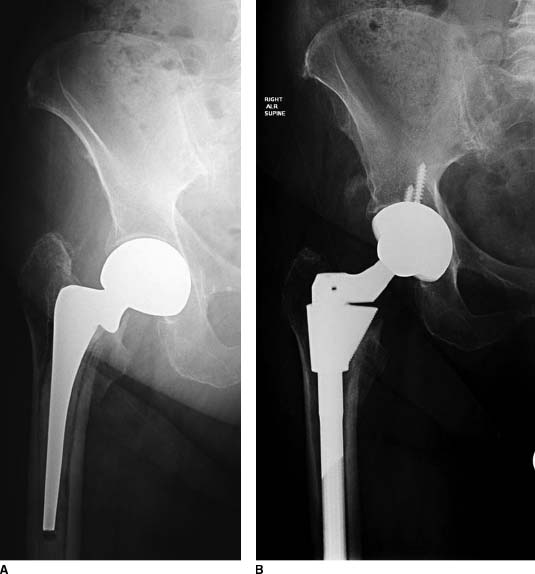
FIGURE 20-4. A,B: Pre- and postoperative radiographs of a patient treated with an S-ROM prosthesis for a loose cemented femoral stem and Paprosky type I bone.Type IIIA defects are treated with an extensively porous-coated stem or a fluted tapered modular stem, while type IIIB defects mostly commonly are now treated with a fluted tapered modular stem which has splines for rotational control and only needs 2 to 3 cm of isthmic fit.
Type II femoral defects are most commonly encountered and demonstrate absent metaphyseal cancellous bone with an intact diaphysis. There is slight proximal varus femoral remodeling. These defects are typically treated with an extensively porous-coated femoral or other standard-length distally fixed implants17,19 (Figs. 20-5 and 20-6).

FIGURE 20-5. Illustration of Paprosky type II femoral defect. (Radiograph from Della Valle CJ, Paprosky WG. The femur in revision total hip arthroplasty evaluation and classification. Clin Orthop Relat Res 2004;(420):55–62.)
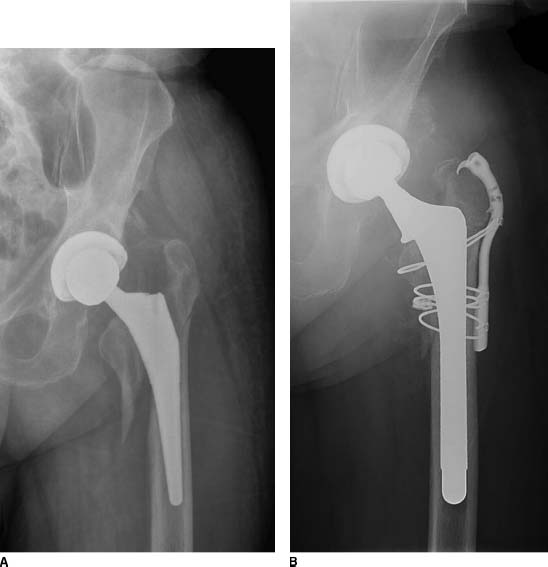
FIGURE 20-6. A,B: Pre- and postoperative radiographs of a patient treated with an extensively porous-coated femoral prosthesis with a claw plate for a Vancouver type AG + B2 periprosthetic fracture. The patient had a Paprosky type II defect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








