Fig. 25.1
Mediastinal emphysema and subcutaneous emphysema of the neck in a diver resulting from a rapid ascent from about 40 m with closed glottis. Plain radiographs (a, b) and CT scan (c). Arrows in image B identify mediastinal gas. (Courtesy of Dr Feletti, own case series)
25.2.2 Pressure Effects on Gas Consumption and Gas Physiology
Scuba equipment supplies the diver with gas at P amb, and gas density therefore increases in direct proportion to P amb as the diver ventures deeper. This has two implications. First, the work of breathing is increased, and this will be further discussed later in this chapter. Second, since more molecules of gas occupy the same volume at higher pressures, gas is consumed at a rate that also increases proportionally to P amb. For example, at 30 msw (4 atm abs) a diver could expect to consume their gas supply twice as fast as at 10 msw (2 atm abs) or four times as fast as at the surface (1 atm abs).
Breathing air (nitrogen 78 %, oxygen 21 %) at elevated P amb results in respiration of oxygen and nitrogen at higher partial pressures than normal. In both cases this can have important consequences.
Oxygen breathed at elevated pressures can result in cerebral irritability and seizures with little or no warning; a phenomenon commonly referred to as ‘cerebral oxygen toxicity’. This is particularly dangerous because loss of consciousness underwater will often lead to drowning. The mechanism of oxygen toxicity is poorly understood, though risk increases with inspired PO2 (PiO2) and the duration of the exposure [5]. There is no clear threshold PiO2 known to be invariably safe, though there is broad consensus that a PiO2 of 1.3 atm abs is relatively safe, even for long duration exposures. This is discussed further below, but in the context of the present narrative, avoidance of a PiO2 of greater than 1.3 atm abs effectively limits the use of air as a breathing gas to depths less than 52 msw (where the P amb = 6.2 atm abs, and the PiO2 when breathing air is therefore 6.2 × 0.21 = 1.3 atm).
Nitrogen breathed at elevated pressures produces a narcotic effect, often referred to as ‘nitrogen narcosis’ that increases progressively with the PiN2. This effect becomes noticeable at depths greater than 30 msw (P amb = 4 atm abs), but it is probably present at shallower depths. There is no universal consensus on a threshold depth beyond which nitrogen narcosis becomes intolerable, but diving with air beyond 40–50 msw is often considered inappropriate for this reason. It should be obvious that cognitive impairment due to nitrogen narcosis could predispose towards incidents [6].
The respiration of gases at elevated pressures results in greater absorption into blood and tissues as predicted by Henry’s law. During ascent, bubbles can form from this accumulated gas. These bubbles may be intravascular (appearing first in the venous system because it is the venous blood ‘draining’ from tissues that is supersaturated), or they may form within the tissues themselves. Depending on the site and profusion of these bubbles, they may cause symptoms of decompression sickness (DCS) (often referred to as ‘the bends’). Venous gas bubbles are routinely detected by ultrasonic methods (venous gas emboli: VGE) after dives that do not result in DCS. However, there is substantial evidence that divers with large right to left shunt pathways (such as a patent foramen ovale) are more vulnerable to developing serious neurological symptoms, implying that VGE can become harmful if they reach the arterial circulation. There are probably many mechanisms by which intravascular and extravascular bubbles can cause pathological effects, including direct mechanical effects, micro-vessel obstruction and activation of coagulation and other complex inflammatory processes. The pathophysiology of this condition is complex and beyond the scope of this chapter. It is described in more detail elsewhere [7, 8]. Symptoms can range from non-serious (such as fatigue, rash, pain) to serious (such as paralysis, cardiopulmonary collapse) [9].
25.3 Recreational Scuba Air Diving
The most common scuba configuration for recreational diving is a single cylinder of compressed air worn on the back with a ‘regulator’ which reduces the cylinder air pressure to P amb and supplies air during inhalation. The diver exhales into the surrounding water. Divers also require a mask (for vision) and fins (for underwater propulsion), and diving in temperate water usually requires the use of thermal protection in the form of a wet suit or dry suit. Most divers wear some form of buoyancy control device, effectively an inflatable water wing to which compressed air can be added (or removed) thus making the diver more (or less) buoyant. A diver wearing an equipment configuration typical of recreational air diving is shown in Fig. 25.2.
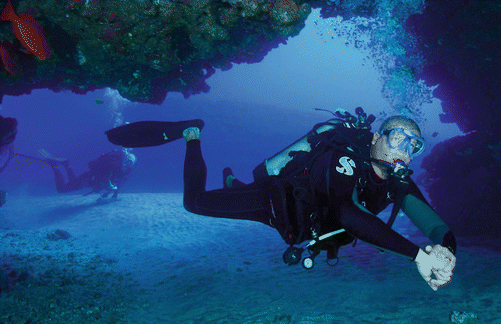

Fig. 25.2
Typical recreational scuba air diver. Note the single cylinder of compressed air and the use of an open-circuit regulator with loss of exhaled gas into the water
Recreational scuba air divers are taught to perform ‘no-decompression’ dives. That is, they are instructed to perform dives where nitrogen absorption into tissues is limited so that a direct ascent to the surface (at a rate not exceeding 9 m/min) is possible at all times during the dive. If the time/depth profile of a dive exceeds so-called no-decompression limits, then decompression stops to allow more time for tissue gas elimination must be made during the ascent. Most divers carry a dive computer that incorporates a timer, a pressure sensor, a microprocessor and an output screen and which continually updates a decompression algorithm and informs the diver how much time they have remaining at the current depth before an ascent with decompression stops becomes necessary. If the no-decompression time is exceeded, then the computer will prescribe the appropriate decompression stops during the ascent. With avoidance of the need for decompression stops and some of the other issues discussed above in mind, recreational diver training organisations usually recommend 40 msw as the maximum depth for scuba air diving.
Despite the seemingly daunting challenges above, recreational scuba air diving is a relatively safe sport. A training database maintained by the Professional Association of Diving Instructors indicates a fatality rate on scuba air training dives of 0.5 deaths per 100,000 dives [10]. Estimates of mortality and nonfatal decompression sickness in non-training scuba air dives are two per 100,000 and ten per 100,000 dives, respectively [11].
25.4 Technical Diving
There is a relatively small group of ‘technical divers’ who perform ‘extreme’ scuba dives beyond the depths and/or durations of typical recreational dives, typically for the purposes of reaching a deep shipwreck or exploring a long-flooded cave. Dives to depths of about 90 msw have become relatively common in this context, and some dives have exceeded 300 msw. Dives in caves lasting well over 12 h have been undertaken.
The challenges involved in the conduct of deep and/or long dives can be predicted from the preceding discussion and include:
- 1.
Reducing the narcotic effect of nitrogen in the respired gas
- 2.
Reducing the toxic effect of oxygen in the respired gas
- 3.
Managing the density of the respired gas
- 4.
Decompressing as quickly as possible while keeping the risk of decompression sickness low
- 5.
Carrying enough gas for very long duration dives
- 6.
Complex logistics including gas supplies and surface support and achieving adequate thermal protection
25.4.1 Mixed Gas Diving: The Pivotal Role of Helium
The first three of these challenges can be met by use of breathing gas mixes containing helium. Helium is a light inert gas that does not produce narcosis at elevated partial pressures. Thus, substituting helium for at least some of the nitrogen in the breathing gas ameliorates both the narcosis and density problems. This typically results in the diver breathing ‘trimix’: a combination of oxygen, helium and nitrogen. Technical divers define the particular mix by stating the fraction of oxygen and helium present. For example, ‘trimix 8:60’ would consist of 8 % oxygen and 60 % helium, and the unstated balance (32 %) is nitrogen.
Nitrogen is rarely substituted completely with helium for several reasons. One is the high cost of helium. This is less of an issue when using a rebreather which recycles exhaled gas (see later), but in open-circuit diving (where all exhaled gas is lost to the water), pure oxygen–helium mixtures (heliox) would be very expensive to use. In addition, some decompression models tend to penalise the use of high helium fractions by mandating longer decompressions. Although this may be unnecessary (see later), it remains a consideration for many divers in planning their gas mixes. Finally, in very deep dives beyond 150 msw, the inclusion of nitrogen in the breathing mix helps to ameliorate the high-pressure neurological syndrome (HPNS) which can cause troublesome tremors and cognitive impairment. HPNS is thought to be due to a pressure effect on excitable membranes, and the dissolution of highly soluble nitrogen into those membranes has an ameliorating effect which is not fully understood [6].
The ‘recipe’ for the optimal trimix for use during the deepest portion of the dive is based on the planned depth, the duration of the dive, the diver’s perception of the maximum safe inspired PO2 and the maximum tolerable narcotic effect. For example, in considering a dive to 90 msw (10 atm abs), the first decision is how much oxygen the mix should contain. Divers will usually aim to breathe as much oxygen as is considered safe, since breathing more oxygen means less inert gas uptake and therefore less decompression. Assuming that a maximum safe PiO2 of 1.3 atm (see earlier) is chosen:
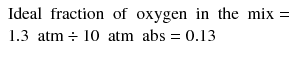 The mix would therefore contain 13 % oxygen for breathing at 90 msw.
The mix would therefore contain 13 % oxygen for breathing at 90 msw.
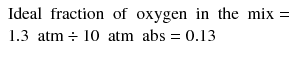
The second decision is the amount of nitrogen in the mix. A common basis for this decision is the degree of narcosis that the diver is prepared to tolerate which in turn is notionally ‘calibrated’ on a comparison with air diving. Thus, assuming a diver is comfortable with the level of narcosis experienced during air diving at 40 msw, they might aim to breathe an equivalent PN2 during the deepest phase of a trimix dive. This is easily calculated by multiplying the fraction of nitrogen in air (0.78) by the ambient pressure at 40 msw (5 atm abs) which gives a PN2 of 3.95 atm. Therefore:
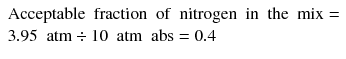 The trimix should therefore contain 40 % nitrogen. This calculation assumes oxygen is not narcotic, but a more conservative approach assuming equal narcotic potency of oxygen and nitrogen yields only a small difference. Having calculated the ideal fractions of oxygen (FO2) and nitrogen (FN2) for the trimix, the helium content (FHe) simply makes up the balance, thus:
The trimix should therefore contain 40 % nitrogen. This calculation assumes oxygen is not narcotic, but a more conservative approach assuming equal narcotic potency of oxygen and nitrogen yields only a small difference. Having calculated the ideal fractions of oxygen (FO2) and nitrogen (FN2) for the trimix, the helium content (FHe) simply makes up the balance, thus:
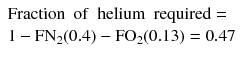 This planning process has determined that an appropriate trimix for a dive to 90 msw is 13 % oxygen, 47 % helium and 40 % nitrogen, designated trimix 13: 47. Another parameter often forgotten in such planning is the density of the resulting gas at the target depth. There is an increasing risk of CO2 retention as the inspired gas density increases, and this result can have life-threatening consequences (see later). It follows that there are sound reasons for ameliorating risk factors for hypercapnia, of which density is one; but there is no clear consensus on the upper density limit. Testing of equipment with gas at a density of 8 g L−1 has seen this figure sometimes cited, but recent (and as yet unpublished) data suggest a risk inflection for CO2 retention around a density of 6.2 g L−1. Calculation of gas density at a target depth is easily achieved based on proportions and adjustment for P amb if given the following densities (g L−1) at 1.0 atm abs: oxygen 1.43, nitrogen 1.25 and helium 0.18. In the above example, trimix 13: 47 at 90 msw (10 atm abs) would have a density of 7.7 g L−1. To comply with a 6.2 g L−1 recommendation, the mix could be adjusted to trimix 13: 60. The respiratory physiology of extreme deep diving is the first of two focus areas treated in more detail later in this chapter.
This planning process has determined that an appropriate trimix for a dive to 90 msw is 13 % oxygen, 47 % helium and 40 % nitrogen, designated trimix 13: 47. Another parameter often forgotten in such planning is the density of the resulting gas at the target depth. There is an increasing risk of CO2 retention as the inspired gas density increases, and this result can have life-threatening consequences (see later). It follows that there are sound reasons for ameliorating risk factors for hypercapnia, of which density is one; but there is no clear consensus on the upper density limit. Testing of equipment with gas at a density of 8 g L−1 has seen this figure sometimes cited, but recent (and as yet unpublished) data suggest a risk inflection for CO2 retention around a density of 6.2 g L−1. Calculation of gas density at a target depth is easily achieved based on proportions and adjustment for P amb if given the following densities (g L−1) at 1.0 atm abs: oxygen 1.43, nitrogen 1.25 and helium 0.18. In the above example, trimix 13: 47 at 90 msw (10 atm abs) would have a density of 7.7 g L−1. To comply with a 6.2 g L−1 recommendation, the mix could be adjusted to trimix 13: 60. The respiratory physiology of extreme deep diving is the first of two focus areas treated in more detail later in this chapter.
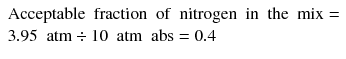
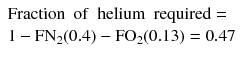
25.4.2 Decompressing as Quickly as Possible While Keeping the Risk of Decompression Sickness Low
Deep dives rapidly accumulate a decompression obligation (the need for decompression stops during the ascent), and it is one of the recognised travails of deep technical diving that more time (often substantially more) may need to be spent decompressing than actually on the bottom. Decompression can be a physically and mentally taxing experience, particularly when conducted in cold water and where there are environmental challenges such as wave action, currents and the onset of darkness. There is a strong motivation to minimise time spent decompressing while at the same time maintaining an acceptably low risk of DCS.
One universally employed strategy to accelerate decompression from deep dives is to increase the fraction of inspired oxygen while maintaining a safe PiO2 as the depth (and ambient pressure) decreases. Breathing a higher FO2 increases the gradient for diffusion of inert gas from tissue to alveoli and thus accelerates inert gas elimination. In many cases, divers choose a breathing mix with less or no helium in the shallower depths during decompression because helium’s low density and non-narcotic properties are no longer necessary. This also saves on the cost of this expensive gas, especially in open-circuit diving. In addition, merely switching from helium to nitrogen is also perceived to accelerate decompression (see later). Thus, divers frequently breathe so-called ‘nitrox’ mixes during decompression. Nitrox is a mix of oxygen and nitrogen with a fraction of oxygen higher than that in air. The mixes are named for the fraction of oxygen (nitrox 32 is 32 % oxygen and 68 % nitrogen). Thus, for example, during ascent a diver might switch to breathing nitrox 32 at 30 msw where the PiO2 would be 1.3 atm. There might be further changes to progressively ‘richer’ oxygen mixes, culminating in a final decompression stop at 3 msw conducted breathing pure (100 %) oxygen (where the PiO2 would also be 1.3 atm).
25.4.3 Carrying Large Gas Supplies or Extending Gas Supply
In order to undertake deep decompression dives, extreme divers must carry much greater supplies of gas or a means of extending a limited supply of gas. For open-circuit divers this inevitably means carrying multiple cylinders of gas during the dive (Fig. 25.3) and, in some cases, staging more cylinders of gas at strategic points on preliminary dives so that they are there for use on the ‘main dive’. The accurate planning of the required gas volumes for deep and/or long dives is one of the most critical skills for a technical diver but will not be described in more detail here.


Fig. 25.3
Technical diver conducting a decompression stop. The two regulators from the diver’s twin, back-mounted cylinders are stowed, and diver is breathing from one of two ‘stage’ cylinders of decompression gas mixtures carried clipped to his harness. The reel of line is connected to a surface marker buoy that the diver deployed so that the surface vessel can track the dive team during a free-floating decompression. Note the diver propulsion vehicle which is not in use and is stowed. (Photo courtesy of A. Hagberg.)
The increasing use of rebreathers for the purpose of reducing gas consumption is arguably the most important development in technical diving over the last decade. A rebreather is a circle circuit containing one-way check valves, one or more counter-lungs, a CO2 absorbent canister and systems for maintaining both the volume of the circuit and an appropriate inspired PO2. Rebreathers are categorised by the nature of the system for maintaining the inspired PO2, and it is beyond the scope of this chapter to detail the operation of all of them. The most prevalent is the so-called electronic closed-circuit rebreather (eCCR). The typical (and simplified) functional layout of one of these devices is shown in Fig. 25.4, and divers wearing rebreathers are shown in Fig. 25.5.
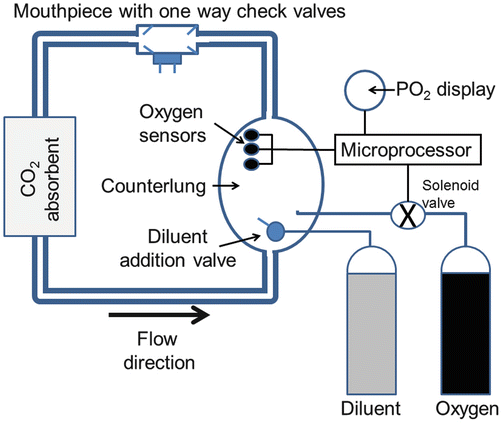


Fig. 25.4
Schematic layout of a typical electronic closed-circuit rebreather. Note, for clarity, the oxygen sensors are portrayed as being located in the counter-lung, but this is never the case. See text for further explanation

Fig. 25.5
Rebreather divers on a decompression station. Note the open-circuit scuba ‘bailout’ cylinders carried by all divers
During use, the diver exhales into the counter-lung through a CO2 absorbent and then inhales from the counter-lung. The one-way check valves in the mouthpiece ensure that flow around the circuit is unidirectional. Three galvanic fuel cells are exposed to the gas in the circuit. These are essentially oxygen-powered batteries that produce an electric current directly proportional to the PO2 to which they are exposed. After calibration against a known PO2, the averaged output of the three cells indicates the circuit PO2, and this is constantly monitored by a microprocessor. A target PiO2 (PO2 ‘set point’) is selected by the diver, and as oxygen consumption reduces the circuit PO2 below this target, the microprocessor opens an electronic solenoid valve to allow oxygen into the circuit to restore and maintain a relatively constant PO2 near the set point. This set point is typically 0.7 atm at the surface and is increased to a higher target (such as 1.3 atm) once the dive is underway.
The volume of the circuit is maintained as Pamb increases during descent by the addition of a diluent gas. When the counter-lung is compressed, the diver will begin to generate a negative pressure in the circuit during inhalation. This opens a mechanical diluent addition valve (Fig. 25.4) allowing diluent gas into the circuit and restoring its volume. For safety reasons, the diluent gas typically contains a fraction of oxygen high enough that the gas is breathable but low enough that the circuit PO2 can still be lowered to the desired set point at the deepest point in the dive. For a dive to less than 50 msw with a PO2 set point of 1.3 atm, air could be used as the diluent gas. Its oxygen fraction of 0.21 still allows a circuit PO2 of ~1.3 atm at 50 msw (ambient pressure of 6 atm abs × 0.21 = 1.26 atm), and at shallower depths the rebreather will add oxygen to maintain the PiO2 at 1.3 atm. Thus, the diver will be breathing a nitrox mix whose oxygen and nitrogen content varies with depth but whose PiO2 remains constant. For a deep dive the diluent gas (usually trimix) is chosen using virtually the same principles as described earlier for mixed-gas diving.
It should be obvious that the crucial advantage of a rebreather is the recycling of exhaled gas thus preserving expensive components like helium. Indeed, the use of diluent gas effectively ends on arrival at the deepest depth provided there is no up-and-down depth variation from that point on. In contrast to open-circuit diving, gas consumption changes little with depth, and the absolute amounts of gas used are vastly smaller.
Another major advantage of rebreathers is the breathing of the optimal safe inspired fraction of oxygen for minimising inert gas uptake and for accelerating decompression throughout the dive. In open-circuit diving, for each gas carried the inspired fraction of oxygen can only be optimal at one depth. Thus, in the example cited earlier, if a PiO2 of 1.3 atm is considered safe, then nitrox 32 breathed during decompression is only an optimal decompression gas at 40 m. On ascent to shallower depth, the PiO2 falls, and the fraction of inspired oxygen is no longer as high as it can safely be (and therefore no longer optimal). In contrast, an eCCR will raise the inspired fraction of oxygen to maintain the 1.3 atm PO2 set point throughout the ascent.
Other rebreather advantages include the breathing of warm, humidified gas and production of few or even no bubbles. The major disadvantages are that the devices are complex, costly and maintenance intensive, provide numerous opportunities for user error and have many potential failure points. Arguably the most significant of these is the oxygen cells which are ‘consuming’ and therefore have a limited and somewhat unpredictable life. Inaccurate data from oxygen cells has been the root cause of many accidents. This potential for failure mandates the requirement for access to open-circuit gas supplies (commonly referred to as ‘bailout’) appropriate for all depths visited and adequate to allow decompression from any point of the dive plan. Planning the carriage of bailout gases is very similar to the planning of an open-circuit deep dive described above. Notwithstanding this precaution, it is perhaps not surprising that crude estimates suggest that rebreather diving is associated with higher mortality (perhaps an order of magnitude higher) than open-circuit diving [12].
25.4.4 Logistics of Technical Diving
Technical diving frequently involves complex logistics to support these ambitious dives. Deep wrecks usually lie in open ocean, and diving them requires large boats for safe and reliable surface support in weather conditions that are rarely optimal. Accurate GPS and sounding equipment are vital, and teams develop considerable skill in accurately dropping a shot line down on to a wreck in deep waters. Divers usually descend and ascend on these shot lines, and purpose-built decompression stages with bars at the depths of the long stops help divers accurately maintain stop depths and allow multiple divers to comfortably occupy the station at the same depth (Fig. 25.5). However, strong currents can complicate such plans and necessitate the use of live boating, where the divers descend from an unanchored surface vessel up-current of the target and complete decompression drifting underneath a surface marker buoy so that the divers do not have to hold onto a shot line against the force of the current. To enhance safety, teams often arrange themselves into bottom diver and support diver roles. Bottom divers actually visit the wreck, and support divers help with surface logistics and visit the bottom divers during decompression. This allows any developing needs to be met and messages to be relayed to the surface.
The exploration of long and frequently deep caves has a different set of logistical challenges. Sequential dives, often very dependent on the use of battery-powered diver propulsion vehicles, are used to penetrate progressively further into the cave and to lay lines into new sections. As there is progress to greater distances, it may become necessary to stage gas supplies at strategic points on the way in before ‘pushing’ the cave further. In this setting, divers may arrange themselves into large teams with specific roles for each individual. Lead divers perform the long pushes. Setup divers may be required to stage gas prior to the dive, and support divers visit the lead divers during their decompression which, as in deep wreck diving, allows any developing needs to be met and messages to be relayed to the surface. In some major cave penetrations, support divers may even install dry underwater habitats (such as an upside-down rainwater tank filled with air) in which the lead divers can actually leave the water while still under pressure in order to rest, eat, drink and warm up.
In both wreck and cave settings, there are numerous logistical considerations which are vitally important but too numerous to discuss here. These include thermal protection and temperature management, hydration and nutrition, gas logistics, medical support and evacuation plans. It should be obvious from this discussion that merely training in the technical diving methods described above is only the start of the process of becoming an exploration-level technical diver.
25.4.5 Current Scope of Technical Diving
The boundary between technical diving and mainstream recreational diving is fluid because technical diving methods and equipment are adopted by and become part of recreational diving [13]. It is difficult to imagine now, but the use of nitrox, presently considered ‘mainstream’ in recreational diving, was viewed as highly technical and fiercely opposed by the recreational diving industry in the early 1990s. In what may prove to be a similar development, there are current plans to develop and promote simplified closed-circuit rebreathers for mainstream recreational diving.
Open-circuit and rebreather trimix dives to a maximum of about 90 msw for bottom times of 30–60 min represent the current state of typical technical diving. Several training agencies specialise in training for this type of diving and several of the large recreational training agencies have also entered this market. Depth record-setting dives (now in excess of 300 msw on open-circuit equipment) typically involve immediate ascent from the maximum depth. However, technical divers are conducting purposeful cave exploration dives in excess of 200-m fresh water (mfw) with substantial bottom times. A notable recent example is the exploration of the Pearse Resurgence cave system in New Zealand to 221 mfw. In addition, some dives of remarkable duration are now being undertaken to explore caves over long distances. The most conspicuous are those conducted by the Woodville Karst Plains Project in northern Florida. This team has conducted exploration out to 7.9 km in Wakulla Springs, a dive requiring 11 h of bottom time at an average depth of 80 mfw, followed by 16 h of decompression.
As technical divers have extended these boundaries deeper and longer, a number of physiological challenges have been pushed into the spotlight, often because of related accidents. This chapter concludes with a more detailed discussion of two such challenges: the respiratory implications of deep diving and issues pertaining to decompression from deep dives.
25.4.6 Respiratory Challenges of Deep Diving
Breathing in the underwater environment invariably requires greater work to achieve lung ventilation than during breathing at the surface. There are multiple potential contributors to this increased work [14].
First, the use of underwater breathing apparatus imposes an external breathing resistance that is not present during normal ventilation. The degree of impediment depends on the type and design of the device. In general, a well-tuned high-quality open-circuit regulator provides less external resistance than a rebreather device because during inhalation, once the demand valve is tripped, the supplied gas flows relatively freely. In addition, exhalation is via a simple mushroom valve to the external environment. In contrast, in a rebreather the diver must generate all the work necessary to move gas through the hoses, valves and CO2 absorbent. Based on evaluation of the influence of work of breathing on dyspnea and CO2 retention (see later), Warkander et al. (1992) proposed that the external work of breathing for UBA should not exceed 1.5–2.0 J/L in the ventilation range of 30–75 L/min [15].
Second, the increase in gas density that occurs as gas is respired at higher ambient pressure increases the resistance to flow through both the diver’s airways and the orifices, hoses and valves of the UBA. Not only does this further increase work of breathing, it also predisposes to the onset of dynamic airway compression during exhalation at much lower flow rates than normal [16]. Since the pressure drop along the airway occurs more quickly when exhaling a dense gas, the equal pressure point will be reached more proximally and at lower flow rates during a forced exhalation. Not surprisingly, the maximum voluntary ventilation is markedly reduced as depth increases [14]. For example, during air breathing, maximum voluntary ventilation is halved at 4 atm abs (30 msw equivalent) compared to 1 atm abs, even when measured with low-resistance respiratory laboratory equipment. In what is probably a subconscious attempt to reduce dynamic airway collapse, divers breathing dense gas tend to increase their expiratory reserve volume. This increases the calibre of small airways by stretching them, but it also shifts tidal breathing to a less favourable part of the lung compliance curve, further increasing the work of breathing [17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








