4.3 Extracellular matrix As already described in Chapter 4.1, extracellular matrix consists basically of three components: connective tissue fibers (collagen and elastic fibers), the ground substance (consisting of glycosaminoglycans (GAGs), and proteoglycans (PGs)), and non-collagenous link proteins. The matrix is produced by various connective tissue cells. The composition of the matrix and the relation between the individual components is determined by the mechanical stress which affects the cells in each case; see Fig. 4.3.1 (Leonhardt 1987). A large quantity of water is associated with the matrix: one of its functions is to enable essential processes, such as the diffusion of nutrients and waste products. Fig. 4.3.1 • Causal histogenesis of connective tissues according to Pauwel’s theory of developmental differentiation. Collagen fibers are white, so collagen-rich tissue is correspondingly white. Collagen fiber “turnover”, the reconstruction phase, normally lasts about 300 to 500 days (Fleischmajer et al. 1990; Currier & Nelson 1992; Van den Berg 2010). In tissues with longer turnover (e.g., intervertebral discs) a yellow to yellow–brown discoloration can be found in older patients; this is true, for example, in the case of the intervertebral discs (Ishii et al. 1991). After water, collagen is the second largest component of connective tissue. It represents approximately 30% of our body protein. Twenty-eight collagen types have so far been identified. For many types, little is known about their specific tasks in the matrix and some are not known at all. The most important collagen types are types I, II, III, and IV. They represent approximately 95% of all collagen. Type I collagen makes up about 80% of these 95% (Leadbetter et al. 1990; Meyer et al. 2007). Collagen fibers occur, for example, when the fibrils spiral around each other in type I and III collagen and a few other types of collagen. In tendons and ligaments, the fibers twist around each other again and form fibrillar bundles. The coils in the collagen are always opposed from one stage to the next – a left-handed helix is followed by a right-handed helix and then a left-handed one again, etc. During traction, the fiber spirals interwind and gain in stability. This is how collagen achieves its enormously high tensile strength of 500–1000 kg/cm2, which is higher than steel. The stability of the collagen is dependent on the physiological cross links. These exist between the individual protein chains within the collagen molecules and as a result of binding of the collagen molecules with each other. The occurrence of cross links is a result of the biochemical bridges of certain amino acids, for the formation of which vitamin C is one of the important ingredients (Grodzinsky 1983; Fleischmajer et al. 1990; Currier & Nelson 1992; Brils et al. 1999a, b; Aaron & Bolander 2005; Van den Berg 2010). Ground substance is found between the crossing collagen fibers. The water bound to it provides for friction-free movement of the fibers against each other. Under pathological circumstances – such as with a loss of ground substance – the collagen fibers get closer to each other and form so-called pathological cross links. These pathological connections reduce the ability of the collagen network to unfold. When examining patients we detect restricted movement (Akeson et al. 1973, 1977, 1987, 1992; Brennan 1989; Van den Berg 2010). In order to loosen the pathological cross links in the tissue, the therapist mobilizes activity with intermittent extension stimuli. This stimulates the fibroblasts to increase synthesis (+ 200%) of collagenase, an enzyme that breaks down the pathological cross links again (Carano & Siciliani 1996).
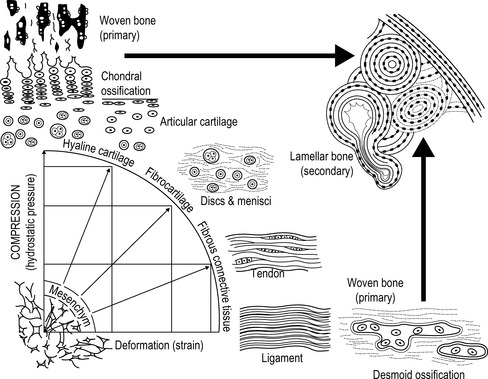
From Leonhard, 1987, with permission.
Collagen fibers
Collagen types
Structure of collagen
Structure of the collagen network
Musculoskeletal Key
Fastest Musculoskeletal Insight Engine









