Michael E. Berend
Extensor Mechanism Complications
Extensor mechanism complications are some of the most common and challenging issues faced by the revision surgeon. The incidence of these complications has varied from 5% to 55%1–12 and has accounted for up to 50% of revision total knee arthroplasty (TKA) surgeries.13–15 Common problems include patellofemoral instability, patellar fracture, patellar component loosening, patellar component failure, patellar clunk syndrome, and infrapatellar or quadriceps tendon rupture with extensor mechanism dysfunction. The success of revision surgery for extensor mechanism complications depends upon accurate identification of the source of the problem. One must recognize that many extensor mechanism complications result from issues outside of the patellofemoral joint. The scope of each of these complications and treatment options will be discussed in detail below.
PATELLOFEMORAL INSTABILITY
The etiology of patellofemoral instability is multifactorial and should be elucidated prior to any treatment for extensor mechanism instability. Instability can occur secondary to soft tissue imbalance, errors in patellar resection or component positioning, or implant design issues. Soft tissue imbalance may result from medial retinacular laxity (Fig. 51-1) or traumatic disruption, weakness of the vastus medialis obliquus (VMO) musculature, or contracture of the lateral retinaculum, all of which will result in excessive lateral forces and lateral patellar tracking. These factors and combined valgus limb alignment may result in an increased functional Q-angle and a relatively lateralized tibial tubercle and may predispose to lateral subluxation or dislocation.6 Weakness of the VMO has been associated with increased patellar subluxation.16 Laskin reported a reduction in patellar tilt as the quadriceps tone returns from 6 to 12 weeks following TKA with patellar tilt decreasing from 41% to 21%, respectively.17
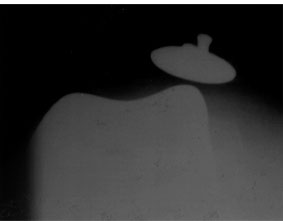
FIGURE 51-1. Sunrise radiograph demonstrating severe lateral subluxation due to medial soft tissue insufficiency and retinacular imbalance.
Asymmetric resurfacing of the patella may also result in maltracking and imbalance. It is important to note the anatomic dimensions of the native patella in which the medial facet is thicker than the lateral facet. A common error in patellar resurfacing is to resect similar amounts of bone from both the medial and lateral facets that results in an asymmetric patella (remaining medial facet thicker than lateral), which will commonly tilt laterally. The goal of patellar resurfacing is restoration of patellar height and a symmetric resection.
It is important to remember that patellofemoral tracking is a composite relationship be-tween femoral, tibial, and patellar component position. Internal rotation of the femoral component relative to the transepicondylar axis or internal rotation of the tibial component relative to the tibial tubercle may predispose to lateral patellar tracking and instability.18 Berger et al.19 examined CT scans of knees with patellofemoral complications following total knee replacement (TKR). They found that the group with patellofemoral complications had excessive combined (tibial plus femoral) internal component rotation. The severity of internal rotation correlated with the severity of the lateral patellar tracking, subluxation, early patellar dislocation, and late prosthesis failure. Similarly, medial shifting of either the femoral or tibial components will create an increased lateral force vector on the patella, enhancing the risk of lateral patellar subluxation.
The median ridge of the patella is the thickest portion in the native patella and the area that articulates in the central portion of the femoral trochlear groove. Intraoperative data indicate that the median ridge is an average of 5.4 mm medial to the center of the patellar bone.20 It is important to place the patellar component medially, with the thickest portion of the implant positioned at the median ridge of the native patella as this has been shown to decrease the need for lateral retinacular release.20 Placement of the patellar component lateral to the desired anatomic position makes capture of the patellar component into the trochlear groove and central tracking more difficult to achieve.
Patellar and femoral component design may influence patellar stability and long-term survivorship. Patellar design features include variations in conformity on the articular surface such as dome-shaped components or anatomic designs with asymmetric facets. Femoral component trochlear groove design features such as the depth of the groove and anatomic valgus orientation may influence patellar stability. Early trochlear groove designs were often shallow and nonanatomic and demonstrated an increased incidence of patellofemoral instability.21 Many “modern” designs have deepened trochlear flanges to attempt to centralize patellar tracking and decrease patellar component loads. Rotating platform mobile-bearing designs may afford potential benefits to the patellofemoral articulation through self-centering of the tibia with the femur, resulting in a decreased Q-angle. Mobile-bearing, metal-backed patellar designs have proven durable in long-term studies with favorable patellar tracking and a 97% survivorship at up to 19-year follow-up duration.22
Treatment of patellofemoral instability is based on its etiology. Intraoperative analysis of patellar tracking is essential and must always be performed following tourniquet release to eliminate its binding effect on the extensor mechanism. Patella tracking should be assessed before capsular closure using the “no-thumb technique.” In cases of extensor mechanism imbalance, a lateral retinacular release, often combined with the advancement of the medial quadriceps muscle (proximal realignment), is indicated. Capsular repair is necessary in cases of early traumatic disruption. Tibial tubercle transfer is considered in cases of severe malalignment. Component revision is reserved for cases with substantial component malposition that are not amenable to simpler realignment procedures.
PATELLAR FRACTURE
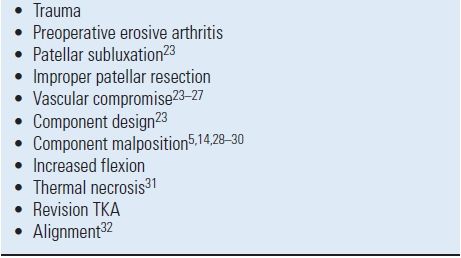
Patellar fractures are an infrequent complication following TKA, although an incidence as high as 21% has been reported. The etiologies of patellar fractures are multifactorial and related to the patient, implant, and technical factors (Table 51.1).28,33–38
TABLE 51.1 Factors Associated with Patellar Fracture
Patellofemoral contact areas are normally eccentric with knee malalignment. With patellofemoral malalignment, both the eccentricity and magnitude of patellofemoral loads are increased,39 enhancing the risk of patellar fracture. Excessive, asymmetric, or inadequate patella resection can all predispose to patellar fracture. As previously mentioned, asymmetric patellar resection, typically resecting too much of the lateral facet, is a common error in TKA. Minimal lateral facet resection, ideally flush with the lateral subchondral bone, is desired to help maintain mechanical strength of the patella. Excessive patellar resection, particularly resection of substantial amounts of the subchondral bone, weakens the patella enhancing the chance of fracture. Patellar osteotomy resulting in an osseous patellar thickness of <15 mm substantially increases anterior patellar strain.40
Conversely, inadequate patellar resection results in thickening of the patella-patellar component composite thickness, with a corresponding increase in quadriceps tension, patellofemoral joint reaction force, and risk of patellar fracture. Using oversized femoral components with excessive anteroposterior diameter or implanting a femoral component in a flexed position similarly increases patellofemoral joint reaction force and enhances the probability of fracture.5,14,28,29,30
Avascular necrosis due to surgical vascular disruption can result in fracture.24–27 Studies of patellar blood supply have delineated both extraosseous and intraosseous systems. The extraosseous system is composed of a peripatellar anastomotic ring supplied by six main arteries. The intraosseous system is comprised of midpatellar, polar (apical), and quadriceps tendon blood supplies. The midpatellar vessels penetrate through the anterior middle one third of the patella and branch both into the proximal and distal poles. The polar vasculature passes through the proximal fat pad posterior to the patellar tendon and enters through the inferior pole of the patella supplying its distal one-half. The quadriceps tendon supplies a small portion of the superior pole of the patella. Substantial disruption of the patellar blood supply occurs during routine TKA.41,42 A medial peripatellar arthrotomy divides the two medial genicular and supreme genicular arteries. Lateral meniscectomy and fat pad excision endanger the lateral inferior genicular artery, while the lateral superior genicular artery is at risk when a lateral retinacular release is required. Postoperative technetium bone scan studies show an increased incidence of “cold” scans if a lateral retinacular release has been performed.41–43
The midpatellar intraosseous blood supply may be compromised during the creation of a large central peg anchoring hole. This type of anchoring hole has also been shown to create increased anterior patellar strain more than smaller peripheral peg configurations.44,45 Fat pad excision further risks avascular necrosis through disruption of the polar intraosseous blood supply. Histologic evaluations of patellar specimens obtained after patellar stress fracture have documented the presence of avascular necrosis.10,32
Errors in implant position and alignment, joint line position, and patellar coverage by the prosthesis risk fracture.32,35 Increased flexion following TKA (creating increased patellofemoral compressive forces), thermal necrosis, and revision TKA have all been reported to have a higher incidence of patellar fracture.31,33,36,46
Fractures can be classified based upon radiographic and functional parameters including displacement, component fixation, and the function of the extensor mechanism. Goldberg et al.35 described a patellar fracture classification system based on the fracture location. In Type I, the fracture occurs through the midbody or superior pole of the patella, not involving the implant, cement, or quadriceps mechanism (Fig. 51-2). Type II fractures of the patella involve those disrupting the quadriceps mechanism or implant-bone-cement composite. Type IIIA fractures occur at the inferior pole of the patella with disruption of the patellar ligament (Fig. 51-3) while Type IIIB fractures are nondisplaced fractures through the inferior pole of the patella (Fig. 51-4).
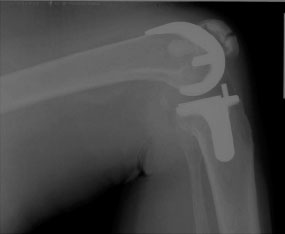
FIGURE 51-2. Lateral radiograph demonstrating a midbody patellar fracture (Type I) without disruption in the extensor mechanism or loss of patellar component fixation.
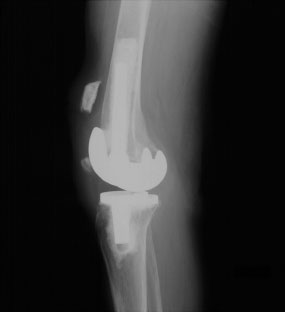
FIGURE 51-3. Lateral radiograph demonstrating inferior pole patella fracture (Type IIIA) with disruption of the extensor mechanism and significant displacement of the patellar fracture.
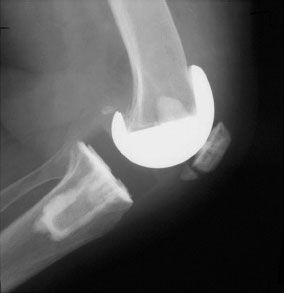
FIGURE 51-4. Lateral radiograph demonstrating an inferior pole patellar fracture (Type IIIB) with no disruption in the extensor mechanism and well-fixed components.
The treatment of the patellar fractures after TKA is guided by fracture displacement, extensor mechanism integrity, and prosthetic component stability.36,47 In some cases, patellar fractures may go unnoticed by the patient and are diagnosed only on routine follow-up radiographs. Fractures that are nondisplaced with an intact extensor mechanism and a stable component can be treated with bracing or casting in extension until the fracture heals with an osseous or fibrous union.28 Displaced fractures with an intact and functioning extensor mechanism with minimal lag and a stable component may also be treated with similar techniques. If the extensor mechanism is disrupted, treatment should be directed at reestablishing extensor mechanism function through direct repair, open surgical fixation of the patellar fracture if bone stock is sufficient, or augmentation with an extensor mechanism allograft. If the component is loose and the patient is symptomatic with an intact extensor mechanism, the component can be removed with improvement in function but often associated with an increased complication rate.48,49
Operative treatment of patellar fractures following TKA has commonly resulted in inferior results with a high rate of complications and reoperations.28,32,47 Ortiguera and Berry47 reported on 12 Type II fractures (associated with disruption of the extensor mechanism) of which 11 were treated operatively. Six knees had complications and five required an additional operative procedure. In the 28 fractures associated with a loose patellar component (Type III), 20 were treated operatively with 9 knees experiencing a complication, and 4 requiring a reoperation.
COMPONENT FAILURE
While component failure of polyethylene patellar designs has been reported,14 this has primarily been a problem of metalbacked patellar designs.5,13,14,30,34,50–54 Failure modes have included excessive polyethylene wear and fracture, polyethylene-metal plate dissociation, peg-plate dissociation, and metal plate fracture. Takeuchi et al. demonstrated in vitro that contact pressures exceeded the yield strength of polyethylene at flexion angles of >60 degrees.55 Thin polyethylene and patellar subluxation (Fig. 51-5) may further increase the risk of accelerated patellar polyethylene wear requiring revision TKA. Metal-backed components may predispose to femoral component metal-on-metal wear if complete polyethylene wear-through occurs (Fig. 51-6) with the creation of excessive amounts of metal debris.56 With metal backing of patellar components, the resultant polyethylene thickness is lessened, predisposing to enhanced wear rates. Polyethylene thickness as minimal as 2.4 mm at the periphery of the metal plate has been observed. In most designs, the metal backing does not totally extend to the polyethylene periphery. As the peripheral polyethylene is loaded, it may thereby deform over the rim of the metal plate and be “cut” by the sharp, nondeforming edge of the metal plate.30,57 The mode of attachment of polyethylene to the metal plate in most designs is a mechanical “gripping” of the plate periphery by the polyethylene, as a chemical bond is not typically present. With excessive peripheral polyethylene wear, the integrity of the polyethylene-plate attachment may be disrupted, allowing the polyethylene to dissociate.30 Fracture of the fixation pegs at their attachment to the metal plate may occur because of high shear stress at this junction, particularly if osseous ingrowth into the fixation pegs occurs without accompanying ingrowth into the metal plate.53,58

FIGURE 51-5. Sunrise radiograph demonstrating lateral subluxation of a metal-backed patella and metal-on-metal wear.
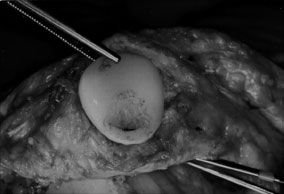
FIGURE 51-6. Intraoperative findings of severe polyethylene wear of a metal-backed patellar component.
Overall, metal-backed patellar components have been associated with increased rates of loosening, revision, and polyethylene wear. Failure of metal-backed patellar components often occurs early, with most reviews reporting failure as early as two years following TKA.13,30,50,53,59 A review of subjects implanted with an AGC PCL-retaining TKA with metal-backed patellar components had a 14% failure rate at an average of 6.8 years.60 This is contrasted with less than a 1% revision rate for all polyethylene patellar components with the same AGC (Biomet, Inc., Warsaw, IN) femoral and tibial designs.23 Dome-shaped metal-backed components with maximized polyethylene thickness and inset implantation techniques have resulted in a lower failure rate than with other metal-backed designs.61
Risk factors for patellar component failure include excessive body weight, enhanced postoperative knee flexion (>115 degrees), high activity levels, as well as male gender.13,30,50,53,59 Other factors associated with increased patellofemoral loading and subsequent component failure include patellar malalignment, increased patella-patellar component composite thickness, use of oversized femoral components, flexion of the femoral component, and alteration of joint line position.
Common clinical signs of patellar component failure include effusion and crepitus which is occasionally audible. Symptoms often occur suddenly after activities that heavily load the patellofemoral joint such as climbing stairs, squatting, or rising from a seated position. Intraoperative findings frequently include black staining of the synovium due to metallosis and wear or fragmentation of the polyethylene. Femoral component wear is not uncommon. Loose porous coating beads or titanium fiber mesh fragments are not unusual and may be found embedded within the polyethylene of the patellar and tibial components.13,51 If revision TKA is required for metal-backed patellar component failure, one should be prepared to revise all three components because of frequent associated damage to the femoral and tibial components.
COMPONENT LOOSENING
Cemented patellar component loosening in TKA has been uncommon, with reported incidence of <2% in most studies (Fig. 51-7).2,9,34,51 The incidence of loosening following cementless patellar resurfacing has been higher, yet variable, with reported loosening rates of 0.6% to 13.5%.1,2,9,62–64 Factors predisposing to loosening include cementation into deficient bone, component malposition, patellar subluxation or fracture, patellar avascular necrosis, osteoporosis, asymmetric patellar bone resection, loosening of other components, and failure of bone ingrowth into porous-coated designs.44,62
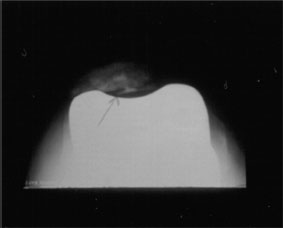
FIGURE 51-7. Sunrise patellar radiograph demonstrating a loose all-polyethylene patellar component.
The natural history of patellar component loosening has been described. Radiographic loosening may not correlate with the need for revision TKA. Berend et al.23 reviewed over 4,000 posterior cruciate-retaining TKAs and found a 4% radiographic loosening rate with only 15 knees (0.3%) requiring additional operative intervention. Patellar loosening was not associated with decreased Knee Society scores when compared to Knee Society scores in knees without a loose patella. Five radiographic features were found to be associated with the process of loosening of an all-polyethylene patellar component (Table 51.2). In this study, patellar loosening was found to be associated with the presence of avascular necrosis and with performance of a lateral retinacular release.
TABLE 51.2 Radiographic Features Associated with Patellar Loosening of a Cemented Single-Peg All-Polyethylene Patellar Components with the AGC TKA
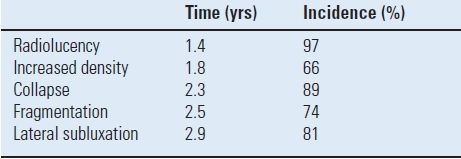
Time for appearance and incidence are presented (n = 4,583 TKR)23.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








