Fig. 2.1
The most common comorbidities found in patients with frozen shoulder. After a multivariate analysis, thyropathy, diabetes, nephrolithiasis, and cancer remained statistically significant, while hypertension was shown to be a protective factor
2.3 Thyropathy
In our data, we found individuals with thyropathy (hypothyroidism, nodules, Hashimoto’s disease, Grave’s disease, or thyroiditis) (Fig. 2.2) were 4 times more likely to develop frozen shoulder (p < 0.001) (Fig. 2.3); however, as the sample consisted of 88 frozen shoulder patients and 229 control patients, further studies should be performed to confirm this data.
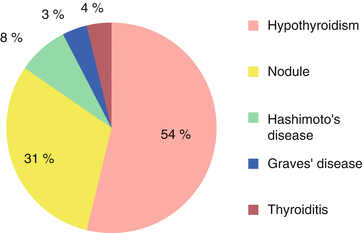
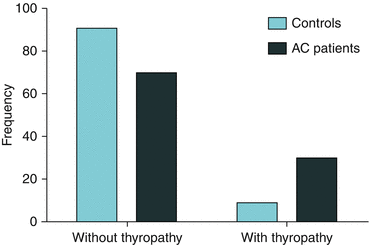

Fig. 2.2
Hypothyroidism represented 54 % of thyroid conditions associated with frozen shoulder

Fig. 2.3
Thyropathy shown as an independent risk factor [p < 0.001; ODDS = 4.37 (95 % CI: 2.01–9.52)] for developing frozen shoulder
Hypothyroidism is known to be risk factor for frozen shoulder. In one study of patients in an endocrinology clinic, frozen shoulder was present in 10.9 % of those with thyroid disorders, 8.8 % with Dupuytren’s contracture, 9.5 % with carpal tunnel syndrome, 4.4 % with limited joint mobility, and 2.9 % with trigger finger [8]. Comparing the medical histories (diabetes, thyroid dysfunction, hypercholesterolemia, and hypertension), drug treatment, and blood tests of 126 frozen should patients with an age-matched control group of 98 patients from an orthopedic foot and ankle clinic and to the regional population disease prevalence registry in Jerusalem, Milgrom et al. [26] concluded that thyroid diseases may be not only be a risk factor for musculoskeletal disorders in general but a specific risk factor for frozen shoulder in women. Bilateral presentation of frozen shoulder was statistically significant (p = 0.014) in the Brazilian population, with the presence of thyroid nodules in comparison to unilateral involvement.
2.4 Diabetes
The diabetic population has a higher incidence of frozen shoulder, between 10 and 36 %, which is 2–4 times greater than in the general population [33]. Insulin-dependent patients are at higher risk [12], and the mean age of onset of disease is younger than that in the general population. There seems to be less pain in frozen shoulder patients with diabetes, but the time course is longer because these patients show less response to treatment [27].
In our study comparing 88 frozen shoulder patients with 229 age- and sex-matched controls, we found diabetes to be an independent significant risk factor (p = 0.006), showing almost 4 times the chance of developing the disease, which is in accordance with the study of Milgrom et al. that found 5 times the risk [5, 26].
Bridgman et al. studied 800 diabetic patients and 600 nondiabetic controls and found the incidence of frozen shoulder to be 10.8 and 2.3 %, respectively (p < 0.05). An association was also found with severity of diabetes: 36 % of frozen shoulder patients were insulin-dependent, whereas only 23 % of the 800 diabetic patients investigated were insulin-dependent ( p < 0,001). Pal et al. [29] found the incidence of frozen shoulder to be 20.4 % of insulin-dependent patients, 18.3 % in non-insulin-dependent diabetic patients, and 5.3 % in the nondiabetic population.
In another study with 778 patients with diabetes [32], the incidence of frozen shoulder was 4.4 % in the diabetic patients, whereas the incidence in the control group was only 0.5 %. The low incidence in this study reflects the strict criteria used for frozen shoulder. The researchers found that 25.7 % of patients in the diabetes group complained of shoulder pain compared with only 5 % in the control group. A longer duration of diabetes was correlated with increased risk of frozen shoulder, although there was no relation with HbA1c levels. Insulin use was not found to be a risk factor.
2.5 Cancer
Cancer has also been pointed to as a risk factor for frozen shoulder. In our study, there was a history of tumor (malignant or benign) in 18.2 % of the frozen shoulder patients (p = 0.017), mainly represented by breast cancer and breast tumor, thyroid cancer, and thyroid nodules (Fig. 2.4).
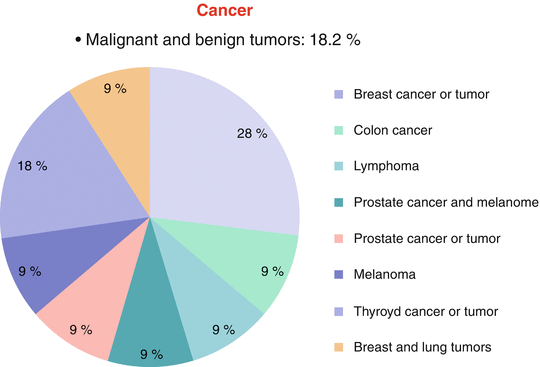

Fig. 2.4
Incidence of malignant and benign tumors in frozen shoulder patients in the Brazilian population, showing prevalence
As survival after breast cancer treatment increases, morbidity in the upper extremity is more frequently seen. Shoulder pain after breast cancer treatment varies between 9 and 68 %, and restricted motion is present in 1–67 % of survivors [16, 36]. Breast cancer treatment is followed by moderately to greatly decreased shoulder flexion and abduction [5, 22].
Primary chest wall tumors may mimic the symptoms of frozen shoulder and may lead to misdiagnosis of the tumor in young patients [9].
2.6 Renal Failure
In patients undergoing long-term hemodialysis, findings of frozen shoulder, as rotator interval obliteration, with magnetic resonance imaging (MRI) correlates with the range limitation of shoulder motion [19]. In our study, renal failure was statistically significant (p = 0.002) in patients with bilateral frozen shoulder in comparison to unilateral presentation of the disease. This may correlate the severity of shoulder involvement with poor renal function, but whether there is an associated mechanism remains unknown.
2.7 Hypertension
A 2014 study showed that there is not a clear correlation between metabolic syndrome markers and frozen shoulder, but, for the first time, an association of hypertension with frozen shoulder resulting from a proinflammatory condition was described [2]. In contradiction, our data showed that hypertension was a protective factor for frozen shoulder (p = 0.02; ODDS = 0.44), perhaps because of increased blood flow or interactions among risk factors. Further studies are needed.
2.8 Parkinson’s Disease
Parkinson’s disease may also correlate with frozen shoulder. It was initially thought that the higher incidence was the result of immobility in later phases of the disease. However, in a study of 150 patients with Parkinson’s disease and 60 controls, Riley et al. [32] showed that shoulder complaints were present in 43 % of those with Parkinson’s disease versus 23 % of the controls. Diagnoses of frozen shoulder were made in 12.7 % of patients with Parkinson’s disease and 1.7 % of the control group. The frozen shoulder group had initial symptoms indicative of akinesia twice as frequently as tremor, whereas the ratio was reversed in the control group. Curiously, in 8 % of the frozen shoulder group, frozen shoulder was the first symptom noted, 0–2 years prior to onset of Parkinson, suggesting a further relation between the two diseases beyond immobility.
2.9 Dupuytren’s Disease
Dupuytren’s disease is believed to have a strong correlation with frozen shoulder. Bunker et al. [7] reported that up to 60 % of patients with idiopathic frozen shoulder have a history of Dupuytren’s disease. In addition, histological findings from samples of shoulder capsule are comparable to samples of from patients with Dupuytren’s disease, showing increased local collagen production, myofibroblasts, and fibroplasia [35].
2.10 Genetics
While strong evidence exists that Dupuytren’s disease has an inheritable component and a racial predilection, the same has not yet been shown in frozen shoulder [25]. A study of 865 monozygotic and 963 dizygotic twins found the prevalence of frozen shoulder to be 11.6 % and the rate of heritability to be 42 % [14].
Future research is needed to confirm whether specific genetic components or environmental factors are responsible. We believe there may be a genetic predisposition to developing frozen shoulder.
2.11 Other Risk Factors
Other risk factors are thought to contribute to the development of frozen shoulder, including ischemic heart disease, use of a pacemaker, stroke, depression and other psychiatric diseases, immobility, neck surgery, cardiac surgery, neurosurgery, smoking, low body mass index, and some medications, such as highly active antiretroviral therapy (HAART) with protease inhibitors for HIV [10, 13, 21], anticonvulsant drugs, and others [1, 11, 28, 39, 42].
A summary of the literature on factors associated with frozen shoulder is provided in Table 2.1.
Table 2.1
Summary of literature on factors associated with frozen shoulder
Associated factor | Incidence of association | Incidence of frozen shoulder | Risk ratio |
|---|---|---|---|
Diabetes mellitus | 29.4 % (Milgrom et al. [26]) | 0.65 % (Yian et al. 2012) to 10.8 % (Bridgman [5]) | 3.08 (1.23–4.98) (Lo et al. [23]) 5.9 for males (4.1–8.4) (Milgrom et al. [26]) 5.0 for females (3.3–7.5) |
Hyperlipidemia | 2.67 (2.36–4.06) (Lo et al. [23])
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




