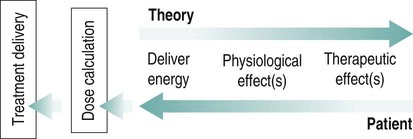
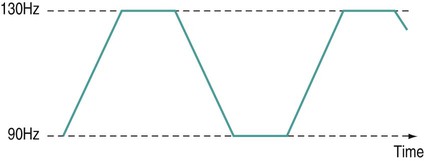
There is a general shift to move away from the term ‘electrotherapy’ toward a more encompassing term of ‘electro-physical agents’ (EPAs). This is largely to be welcomed in that electrotherapy, in the strictest sense of the term, would only apply to those modalities which involved the delivery of electrical, and possibly also electromagnetic, energies (e.g. transcutaneous electrical nerve stimulation (TENS), interferential therapy (IFT)). Ultrasound, light, vibration and various heat therapies, for example, would not fit this more narrow definition. As a term, EPA is certainly more inclusive and thus a more accurate reflection of the wide range of modalities employed in physiotherapy (Watson 2010). A general shift away from the term electrotherapy and towards a more common use of EPAs is anticipated over the next few years. The term electrotherapy in the context of this chapter will be used in its broad, inclusive sense rather than as a strict definition of the energy applied. The aim of this chapter is to enable the reader to identify the key issues in electrotherapy, using commonly employed modalities as examples. It does not purport to fully examine or explain the evidence for every modality, and there are many ‘modalities’ that will hardly be mentioned. This does not mean that they are unimportant or worthless: it is a realistic reflection of the complexity of modern electrotherapy practice and the limits of what can be achieved in a single chapter. Further details are available in the standard texts (e.g. Robertson et al. 2006; Watson 2008b; Belanger 2010) and reference will be made in this chapter to further useful research articles and resources. In terms of clinical practice, the most widely used modalities in the UK are ultrasound, IFT and TENS, with pulsed shortwave with laser and neuromuscular electrical stimulation (NMES) following close behind. Microcurrent therapy, shockwave therapy, low intensity pulsed ultrasound (LIPUS) and some new radio frequency (RF) applications have been added to this chapter as evidenced and emerging interventions. Electrotherapy modalities follow a very straightforward model that is presented below. The model (Figure 19.1) identifies that the delivery of energy from a machine or device is the starting point of the intervention. The energy delivery to the tissues results in a change in one or more physiological events – some of which are very specific while others are multifaceted or more general. The capacity of the applied energy to influence physiological events is key to the process. The physiological shift that results from the energy delivery is used in practice to generate what is commonly referred to as therapeutic effects. Having identified the modality that is best able to achieve the effects required, the next clinical stage is to make a ‘dose’ selection. Not only is it critical to apply the right modality, but it needs to be applied at the appropriate ‘dose’ in order for maximal benefit to be achieved. There is a substantial and growing body of evidence that the same modality can be applied at different doses and the results will be different (Watson 2010). An example might be the use of ultrasound energy. Applied at a low ‘therapeutic’ dose, it can stimulate tissue repair and healing. Applied at a much higher dose (high intensity focussed ultrasound (HIFU) ) it can be used to ablate tumour tissue. The energy form is the same, but by varying the applied ‘dose’ the outcome is clearly different. Given the research evidence, there appear to be several aspects to this issue. Using a very straightforward model, there is substantial evidence, for example, that there is an amplitude or strength window. An energy delivered at a particular amplitude has a beneficial effect while the same energy at a lower amplitude may have no demonstrable effect (too low) or a tissue destruction effect (too high in therapy terms). Laser therapy offers an obvious example: one level will produce a distinct cellular response while a higher dose can be considered to be destructive. Karu (1987) demonstrated and reported these principles related to laser energy and the research produced since have served to reinforce the concept (e.g. Vinck et al. 2003). Further examples of amplitude windows can be seen easily in the work of Hill et al. (2002), Reher et al. (2002), Miller and Gies (1998), Cleary (1987) and Pereira et al. (2002), and have been more extensively reviewed in Watson (2010). Along similar lines, ‘frequency windows’ are also apparent. A modality applied at a specific frequency or pulsing regime might have a measurable benefit, while the same modality applied using a different pulsing profile may not appear to achieve equivalent results. Examples can be found in many articles, including Young and Dyson (1990a), Young and Dyson (1990b) and Sontag (2000). Electrical stimulation frequency windows have been proposed and there is clinical and laboratory evidence to suggest that there are frequency-dependent responses in clinical practice. TENS applied at frequency X appears to have a different outcome to TENS applied at frequency Y in an equivalent patient population. Studies by Sluka et al. (2006), Han et al. (1991) and Palmer et al. (1999) illustrate the point. Assuming that there are likely to be more than two variables to the real world model, some complex further work needs to be invoked. There is almost certainly an energy or time-based window (e.g. Hill et al. 2002) and then another factor based on treatment frequency (number of sessions a week or treatment intervals). Work continues to identify the more and less critical parameters for each modality across a range of clinical presentations and a more detailed review of these concepts can be found in Watson (2010). No matter which classification of the numerous electrotherapy modalities is adopted, it can easily be criticised. The groupings used in Figure 19.2 are one way of looking at the scope of electrotherapy, although it is not presented as the ‘right’ model. The thermal modalities group includes various forms of heating that have been used for many years in therapy, including infrared therapy, conductive heating, wax therapy, hot packs and the ‘deeper’ heating modalities – shortwave diathermy (SWD), other RF applications and microwave diathermy (MWD). The use of the heating modalities has diminished in clinical practice over recent years and much as some of the interventions employed in the past may lack evidence, there are compelling reasons to keep heat therapies in the clinical repertoire and there are areas where the use of heat-based therapies is likely to re-emerge as a strongly evidence-based intervention. Examples of modern heat therapy applications include Michlovitz et al. (2004), Usuba et al. (2006), Mayer et al. (2006), and Leung and Cheung (2008). Assuming that the majority of ES modalities work by means of nerve activation, a brief examination of how this is achieved would be beneficial. A nerve in its resting state is said to be ‘polarised’. When an action potential is transmitted along a nerve, the membrane at the point of the action potential is momentarily depolarised before returning to its normal state (repolarisation). Essentially, the employment of an electrical current or pulse is as a means of initiating an action potential along the course of the nerve. Once the action potential has been initiated by this exogenous (external to the body) signal, then it will continue along the nerve (whether sensory or motor) in the normal fashion: the electrical stimulator is simply an initiator of the activity. If the nerve is stimulated ten times a second, then there will be ten action potentials a second initiated. If stimulated 100 times a second, predictably, it will fire 100 times a second. There are some constraints to this relationship based on refractory periods and threshold potentials which are beyond the scope of this chapter but are usefully reviewed in most standard electrotherapy texts (Robertson et al. 2006; Watson 2008b). TENS is a method of electrical stimulation which aims primarily to provide a degree of pain relief (symptomatic) by specifically exciting sensory nerves and thereby stimulating either the pain gate mechanism and/or the opioid system (Walsh 1997; Sluka and Walsh 2003; Johnson 2008). Strictly speaking, any form of electrical stimulation applied with surface electrodes that stimulates nerves can be referred to as TENS but in clinical practice the term is most commonly employed in the context identified above. The technique is non-invasive and has few side effects when compared with drug therapy. Modern TENS devices may be analogue (Figure 19.3a,b) or digital (Figure 19.3c,d) in design. Although their outputs are essentially the same, their control systems differ. TENS is normally included in the multimodal stimulators (Figure 19.3f). Most TENS applications are now made using self-adhesive, pre-gelled electrodes (Figure 19.3e) which have several advantages, including a lower allergy incidence, a reduced cross-infection risk, easier application and lower overall cost, although some practitioners retain the older, impregnated carbon rubber electrode systems. Specialist TENS variations include ‘maternity’ TENS (Figure 19.3g), which have a simple to operate ‘boost’ function. Pain relief by means of the pain gate mechanism primarily involves activation (excitation) of the Aβ sensory fibres, thus reducing the transmission of the noxious stimulus from the ‘c’ fibres through the spinal cord and on to the higher centres. The Aβ fibres appear to respond preferentially when stimulated at a relatively high rate (in the order of 80 or 90–130 Hz). It is difficult to find support for the concept that there is a single frequency that works best for every patient, but this range appears to cover the majority of individuals (Walsh 1997). This TENS mode is delivered with high frequency (traditional/normal) TENS. An alternative approach is to stimulate the Aδ fibres which respond preferentially to a much lower rate of stimulation (in the order of 2–5 Hz), which will activate the opioid mechanisms and provide pain relief by causing the release of an endogenous opiate (encephalin) in the spinal cord which will reduce the activation of the noxious sensory pathways (Han et al. 1991; Walsh 1997; Sluka et al. 2006). This TENS mode is delivered with low-frequency (acupuncture (AcuTENS) ) TENS. Traditional TENS usually uses stimulation at a relatively high frequency (80 or 90–130 Hz) and employs a relatively narrow pulse width (often used at about 100 µs though, as identified above, there is less support for manipulation of the pulse width in the current research literature and the use of a fixed pulse duration of around 200 µs may be the most efficient). The stimulation is delivered at ‘normal’ intensity (see below). Thirty minutes is probably the minimally effective time, but it can be delivered for as long as needed. The main pain relief is achieved during the stimulation, with a limited ‘carry over’ effect, i.e. pain relief after the machine has been switched off (Chesterton et al. 2002). When using AcuTENS, TENS is used at a lower stimulation frequency (2–5 Hz) with longer duration pulses (200–250 µs). The intensity employed will usually need to be greater than with the traditional TENS – a definite, strong sensation but still one that is not painful (see below). A minimally useful stimulation of 30 minutes should be delivered. It takes some time for the opioid levels to build up with this type of TENS and hence the onset of pain relief may be slower than with the traditional mode. Once sufficient opioid has been released, however, it will keep on working after cessation of the stimulation. Many patients find that stimulation at this low frequency at intervals throughout the day is an effective strategy. The ‘carry over’ effect may last for several hours in the clinical setting, though timeframes of rather more limited duration have been demonstrated by Chesterton et al. (2002). Brief intense TENS is a mode that can be employed to achieve rapid pain relief, but some patients may find the strength of the stimulation too intense and will not tolerate it for sufficient duration to make the treatment worthwhile. The pulse frequency applied is high (in the 90–130 Hz band) and the pulse width is also high (≥200 µs). The current is delivered at, or close to, the tolerance level for the patient such that they would not want the machine turned up any higher. In this way, the energy delivery to the patients is relatively high when compared with the other approaches. It is suggested that 15–30 minutes at this stimulation level is the most that would normally be used. Pain relief onset is rapid and marked if the patient can cope with the stimulation intensity (Walsh 1997; Sluka and Walsh 2003). It is not possible to describe the treatment current strength in terms of how many (milli)amps should be applied. The most effective intensity management appears to be related to what the patient feels during the stimulation and this may vary from session to session, though will tend to be fairly consistent for any individual patient. As a general guide, it appears to be effective to go for a ‘definitely there but not painful’ level for the normal (high) TENS and a ‘strong but not painful’ level for the acupuncture (low) mode (Sluka and Walsh 2003). Figure 19.5 illustrates these settings on a ‘subjective’ scale. Some evidence (e.g. Bjordal et al. 2003; Aarskog et al. 2007) suggests that stronger stimulation might be more effective for clinical pain states. • stimulation of appropriate nerve root(s); • stimulate the peripheral nerve (proximal to the pain); • stimulate motor point (innervated by an appropriate nerve root level); • stimulate trigger point(s) or acupuncture point(s); • stimulate the appropriate dermatome, myotome or sclerotome. It is beyond the scope of this chapter to detail specific electrode combinations for specific clinical problems and, in any case, would probably be inappropriate to do so. The TENS literature covers electrode placement in some detail and the interested reader is referred to useful specific texts in this context (Walsh 1997; Johnson 2008). Numerous systematic and Cochrane Reviews have been published relating to the application of TENS for several different clinical pain groups (e.g. Rutjes et al. 2009; Walsh et al. 2009). Many of these come to an ‘inconclusive’ conclusion, though this may be related to dose-related issues (therapeutic windows) (Johnson and Martinson 2007; Watson 2010) and methodological limitations of the research rather than the failure of TENS to have a significant effect. The basic principle of IFT is to utilise the strong physiological effects of low frequency (≅<250 pps) electrical stimulation of muscle and nerve tissues without the associated pain encountered with low frequency stimulation (Watson 2000; Palmer and Martin 2002). IFT is delivered using either dedicated main s-powered interferential devices (Figure 19.6), portable (battery-powered) devices (Figure 19.7) or multimode units that include IFT stimulation among several other treatment modes (Figure 19.8). The effects of tissue stimulation with these ‘medium frequency’ currents (medium frequency in electromedical terms is usually considered to be 1 kHz–100 kHz) is not fully understood and while it is likely to have an effect, little detail is currently known though it is assumed not to directly stimulate nerve. Ward (2009) recently reviewed the key issues with medium frequency currents. The exact frequency of the resultant interference (or beat frequency) can be controlled by the input frequencies. If, for example, one current was at 4000 Hz and its companion current at 3900 Hz, the resultant beat frequency would be at 100 Hz carried on a medium frequency 3950 Hz amplitude modulated current (Figure 19.9). The use of two-pole IFT stimulation is made possible by electronic manipulation of the currents – the interference occurs within the machine instead of in the tissues. There is no known physiological difference between the effects of IFT produced with two- or four-electrode systems; in fact, the pre-modulated currents can be considered superior in clinical effectiveness terms (e.g. Ozcan et al. 2004). The key difference is that with a four-pole application the interference is generated in the tissues and with a two-pole treatment, the current is ‘pre-modulated’, i.e. the interference is generated within the machine unit. Nerves will accommodate to a constant signal and a sweep (or gradually changing frequency) is often used to overcome this problem. The principle of using the sweep is that the machine is set to automatically vary the effective stimulation frequency using either pre-set or user-set sweep ranges. The sweep range employed should be appropriate to the desired physiological effects (see below). It has been repeatedly demonstrated that ‘wide’ sweep ranges are ineffective in the clinical environment. The clinical advantage of the sweep treatment application, beyond that of minimising the accommodation effects, are that a range of treatment frequencies can be automatically applied (Watson 2000). The pattern of the sweep makes a significant difference to the stimulation received by the patient. Most machines offer several sweep patterns, though there is very limited ‘evidence’ to justify some of these options. In the classic ‘triangular’ sweep pattern, the machine gradually changes from the base to the top frequency, usually over a time period of six seconds, though some machines also offer one- or three-second options. In the example illustrated (Figure 19.10), the machine is set to sweep from 90 to 130 Hz, employing a triangular sweep pattern. All frequencies between the base and top frequencies are delivered in equal proportion. Other patterns of sweep can be produced on many machines, for example a rectangular (or step-like) sweep. This produces a very different stimulation pattern in that the base and top frequencies are set, but the machine then ‘switches’ between these two specific frequencies rather than gradually changing from one to the other. Figure 19.11 illustrates the effect of setting a 90–130 Hz rectangular sweep. There is a clear difference between these examples, even though the same ‘numbers’ are set: one will deliver a full range of stimulation frequencies between the set frequency levels and the other will switch from one frequency to the other. There are numerous other variations on this theme and the ‘trapeziodal’ sweep (Figure 19.12) is effectively a combination of these two. It has been suggested that IFT works in a ‘special way’ because it is ‘interferential’ as opposed to ‘normal’ stimulation. The evidence for this special effect is lacking and it is most likely that IFT is just another means by which peripheral nerves can be stimulated. Many regard it as more acceptable than other forms of electrical stimulation as it generates less (skin) discomfort (e.g Shanahan et al. 2006). The are four main clinical applications for which IFT appears to be used: Electrical stimulation for pain relief has widespread clinical use, though the direct research evidence for the use of IFT in this role is limited. Logically one could use the higher frequencies (90–130 Hz) to stimulate the pain gate mechanisms and thereby mask the pain symptoms. Alternatively, stimulation with lower frequencies (2–5 Hz) can be used to activate the opioid mechanisms, again providing a degree of relief. These two different modes of action can be explained physiologically and will have different latent periods and varying duration of effect (same as for TENS). It remains possible that pain relief may be achieved by stimulation of the reticular formation at frequencies of 10–25 Hz or by blocking C fibre transmission at >50 Hz. Although both of these latter mechanisms have been proposed with IFT, neither have been categorically demonstrated (Palmer and Martin 2008). A good number of studies (e.g. Johnson and Tabasam 2003; Hurley et al. 2004; McManus et al. 2006; Jorge et al. 2006; Walker et al. 2006; Lau et al. 2008; Fuentes et al. 2010) provide substantive evidence for a pain relief effect of IFT. Stimulation of the motor nerves can be achieved with a wide range of frequencies. Clearly, stimulation at low frequency (e.g. 1 Hz) will result in a series of twitches, while stimulation at 50 Hz will result in a tetanic contraction. There is limited evidence at present for the ‘strengthening’ effect of IFT (evidence does exist for some other forms of electrical stimulation), though the article by Bircan et al. (2002) suggests that it might be a possibility. On the basis of the current evidence, the contraction brought about by IFT is no ‘better’ than would be achieved by active exercise, though there are clinical circumstances where assisted contraction is beneficial. For example, to assist the patient to appreciate the muscle work required (similar to surged Faradism used previously, but much less uncomfortable). For patients who cannot generate useful voluntary contraction, IFT may be beneficial as it would be for those who, for whatever reason, find active exercise difficult. There is no evidence that has demonstrated a significant benefit of IFT over active exercise. The choice of treatment parameters will depend on the desired effect. The most effective motor nerve stimulation range with IFT appears to lie between approximately 10 and 20 Hz (maybe between 10 and 25 Hz). There is very little, if any, quality evidence demonstrating a direct effect of IFT on local blood flow changes. Most of the work that has been done involves laboratory experimentation on animals or asymptomatic subjects, and most blood flow measurements are superficial, i.e. skin blood flow. Whether IFT is actually capable of generating a change (increase) in blood flow at depth remains questionable. The elegant study by Noble et al. (2000) demonstrated vascular changes at 10–20 Hz, though they were unable to identify clearly the mechanism for this change. The stimulation was applied via suction electrodes and the outcome could, therefore, be a result of the suction rather than the electrical stimulation, though this is largely negated by virtue of the fact that other stimulation frequencies were also delivered with the suction electrodes without significant flow changes. The most likely mechanism therefore is via muscle stimulation effects (IFT causing muscle contraction which brings about a local metabolic and thus vascular change). The possibility that the IFT is acting as an inhibitor of sympathetic activity remains a theoretical possibility rather than an established mechanism. A study by Jarit et al. (2003) demonstrated a change in oedema following knee surgery in an IFT group; however, a study by Christie and Willoughby (1990) failed to demonstrate a significant benefit on ankle oedema following fracture and surgery. The treatment parameters employed are unlikely to be effective given the information now available. If IFT has a capacity to influence oedema, the current evidence and physiological knowledge would suggest that a combination of pain relief (allowing more movement), muscle stimulation (above) and enhanced local blood flow (above) is the most likely combination to be effective and thus 10–20 Hz stimulation around the largest local muscle group is probably the most effective approach. Stimulation can be applied using several different electrode systems (Figures 19.13 and 19.14). Pad electrodes and sponge covers are commonly employed; electroconductive gel is an effective alternative. The sponges should be thoroughly wet to ensure even current distribution. Self-adhesive pad electrodes are also available (similar to the newer TENS electrodes) and in the view of many practitioners make IFT application easier. The suction electrode application method has been in use for several years and while it is useful, especially for larger body areas like the shoulder girdle, trunk, hip and knee, it does not appear to provide any therapeutic advantage over pad electrodes. Care should be taken with regard to the maintenance of electrodes, electrode covers and associated infection risks (Lambert et al. 2000). There has been a significant increase in the use of portable or mains-powered muscle stimulators which are effectively the modern replacement for Faradism. Figure 19.15 illustrates examples of dedicated battery-powered and multimodal mains-powered devices. It is suggested, with a growing body of evidence, that gains in strength, endurance capacity and function can be achieved with these types of stimulation. Much of the early work has been conducted in laboratory studies and also with athletes rather than typical patient groups. There are recent articles, however, that have demonstrated significant clinical benefit with patient groups, including strengthening of peripheral musculature (Talbot et al. 2003; Callaghan and Oldham 2004; Stevens et al. 2004; Lyons et al. 2005), work with shoulder problems in stroke patients (Chantraine et al. 1999; Ada and Foongchomcheay 2002), chronic obstructive pulmonary disease (COPD)/cardiac patients (Neder et al. 2002; Zanotti et al. 2003; Vivodtzev et al. 2006) and various forms of incontinence (Indrekvam et al. 2001; Amaro et al. 2003; Barroso et al. 2004). Useful reviews of this field of intervention are included in McDonaugh (2008), Robertson et al. (2006) and Lake (1992). A further whole branch of neuromuscular-based stimulation is the so called functional electrical stimulation (FES). In this branch of electrotherapy, the explicit intent of the stimulation is to achieve controlled muscle activation in order to facilitate some aspect of functional activity. The range of applications is growing swiftly, especially with the recent advances in computer-controlled stimulators. One of the early, and most successful areas, is the dropped foot stimulator (Taylor et al. 1999; Sheffler et al. 2006) which assists patients – most often following a stroke – to achieve ankle dorsiflexion during the swing phase of gait utilising stimulation of the anterior tibial nerve. Modern devices incorporate a range of foot switches or pressure detectors which enable stimulation to be active at exactly the right part of the gait cycle (Figure 19.16). Other forms of FES include standing and gross walking activity with paraplegic patients which is also making significant gains with computerised control systems. It is interesting in that it appears to break all the classification ‘rules’ that were identified earlier in the chapter. It is not delivered with the intention of stimulating nerves, but rather plays to the bioelectric environment of the tissues, and therefore has a primary effect in terms of tissue repair (Watson 2006a; Poltawski and Watson 2009). The general characteristics of this type of therapy are that they utilise a direct current (pulsed or continuous) delivered at a very low amplitude (literally in the microamperage (millionths of an Amp) range) which is usually subsensory from the patient perspective. This type of therapy has already been shown to be effective in several clinical areas – most notably fracture repair (Simonis et al. 2003; Ciombor and Aaron 2005) and healing of open wounds (Watson 1996; Evans et al. 2001; Watson 2008a) though soft tissue repair research is now evolving and showing strong future potential. The use of this therapy in tissue injury treatment has been reviewed recently (Poltawski and Watson 2009). Microcurrent therapy devices appear to be most effectively employed when used for hours a day (rather than minutes a week in the clinic) and therefore home-based therapy with small, inexpensive, portable devices (Figure 19.17) is a likely way forward. Microcurrent therapy can, of course, be delivered using mains-powered and multimodal devices (e.g. Chatanooga Intelect and Gymna 400) and it has yet to be clearly determined whether the small portable battery-powered home-based machines or the clinic-based ones will become the most widely employed. It is not possible in a general chapter such as this to identify all the possible combinations and variations. The interested reader might try investigating the major texts in the area (Robertson et al. 2006; Watson 2008b; Belanger 2010). Try the current literature (though many of these ‘new’ devices are yet to have specific research published with regard to their efficacy) or utilise web-based resources (though as with any web-based material, one needs to be both critical and selective with regards the information accepted).
Electrotherapy
Introduction
Electrotherapy versus electrophysical agents
Scope
Model of electrotherapy
Therapeutic windows
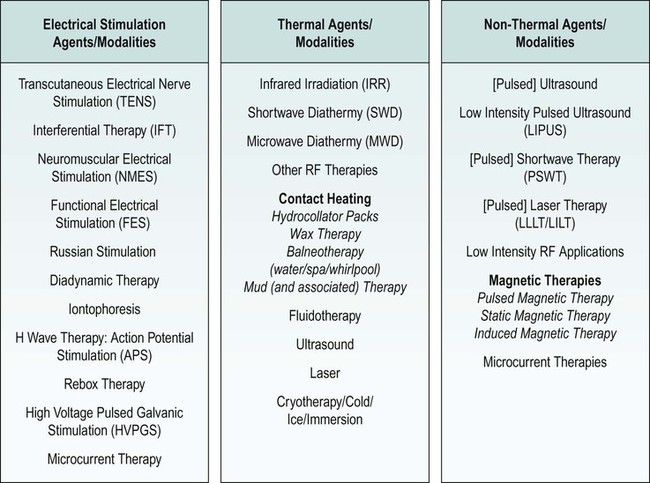
Electrotherapy modality grouping
Electrical stimulation modalities
General principles of electrical stimulation
Nerve action potentials
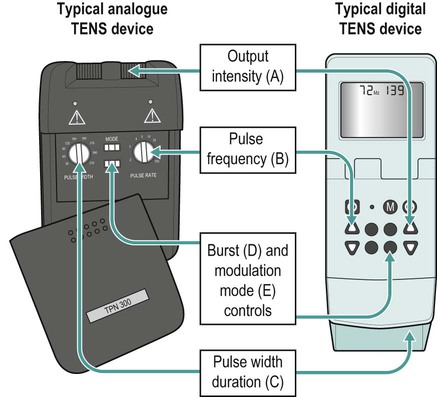
Transcutaneous electrical nerve stimulation (TENS)
Mechanism of action
Traditional TENS (hi-TENS, normal TENS)
Acupuncture TENS (lo-TENS, AcuTENS)
Brief intense TENS
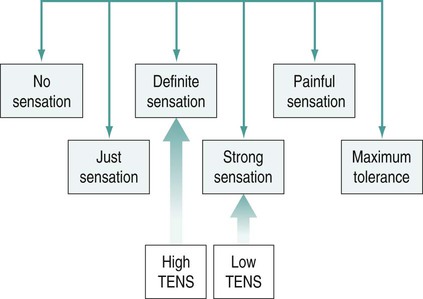
Stimulation intensity
Electrode placement
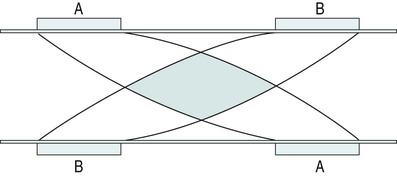
Interferential therapy (IFT)
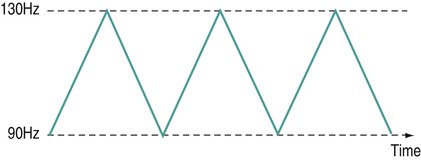
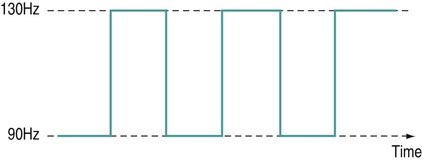
Frequency sweep
Physiological effects and clinical applications
Pain relief
Muscle stimulation
Blood flow
Oedema
Treatment parameters


Muscle stimulation modalities
Microcurrent therapy
Other forms of electrical stimulation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine