45 Key Points 1. Optimal outcome assessment for SCI clinical trials is widely debated. Although much of the SCI literature focuses on neurological status, motor and sensory function, and functional capacity, electrophysiological assessments offer the potential to detect subclinical changes in neurological functioning objectively and sensitively. 2. Two electrophysiological assessment tools are highlighted in this review. Electrical perceptual threshold testing measures minimally detectable sensations, and the method is well tolerated by individuals, can be performed in less time than the American Spinal Injury Association Impairment Scale (AIS) sensory exam, and provides a simple, reproducible, quantitative sensory assessment of the level and degree of impairment in SCI. The brain motor control assessment analyzes suprasegmental control of segmental motor activity using EMG. The protocol response is quantitative and objective, and it provides measurable parameters of the neural components of each assessed motor task. 3. The SCI literature reflects a growing consensus that current outcome measures for recovery after SCI are insufficient. Most researchers agree that electrophysiological tests offer the best opportunity for enhancing assessments, especially as a complement to AIS; however, until there is more work done on the standardization of the acquisition and analysis of data, clinical applicability of these tests will be limited. Awareness of spinal cord injury (SCI) and its consequences is becoming more widespread. Recently, the prevalence of SCI in the United States was estimated to be ∼ 1.27 million, with 11,000 new cases reported annually.1 Resultant impairments can include motor and sensory deficits, ranging from complete paralysis to mildly disturbed gait or balance; autonomic dysfunction, including cardiovascular and respiratory disturbances; bowel, bladder, and sexual dysfunction; and a significant number of secondary complications, such as pressure sores, osteoporosis, and depression.2 Fortunately, advances in the treatment of experimental SCI are being made at an accelerated pace.3,4 Developments in neuroprotection, regeneration, and rehabilitation support the field’s general optimism and provide realistic hope that a cure is imminent. Although effectively treating experimental SCI in animals is an enormously important task, translating this research into clinically feasible interventions for humans will not necessarily follow. Translation of medical research into the clinical sector is a difficult task for any field. First, anatomical, physiological, and sheer size differences between experimental animal models and humans can lead to vastly distinct outcomes when a treatment is translated.5 To account for this expected variation, the medical community requires rigorous testing and infallible findings from preliminary research before an intervention can be successfully translated. As such, most clinical treatments are safe, reliable, and effective, but innovation is tremendously challenging. The issue of running clinical trials for SCI therapies has been well documented.2,5–7 Briefly, to translate a therapy from bench to bedside, researchers must (1) establish its efficacy in animal models, (2) overcome general model-to-model obstacles like size and anatomy, (3) verify safety and potential benefit despite confounding variables, (4) recruit an appropriate sample of human participants to minimize injury variability without creating a group so specific that results won’t be generalizable to the SCI population at large, and (5) demonstrate that the treatment is efficacious.5,7 This chapter addresses only the final step of this demanding process by outlining the issue of outcomes measures, explaining how currently available measures for SCI are insufficient, and providing suggestions for their improvement, specifically, encouraging the refinement and expanded use of electrophysiological measures, such as the electrical perceptual threshold (EPT) test and the brain motor control assessment (BMCA) protocol. The objective and valid conduct of successful clinical studies, and, eventually, the effective choice of therapeutic approach and rehabilitation planning, requires the development of appropriate tools and measures.6,8 However, validly and reliably measuring any type of behavior is difficult because variation in baseline, recovery, and confounding factors produces unique patterns of activity.9 Evaluating neuronal function presents additional challenges; the intricacy, organization, and widespread involvement of the nervous system make it nearly impossible to directly map anatomy to function.2,6 Assessing the capacity of the spinal cord usually involves analyzing locomotor behavior, which adds yet another layer of complexity because it is a multi-faceted behavior involving several distinct functions and physiological systems.10 Particularly challenging is the establishment of reliable baseline measures immediately following SCI because it usually results from trauma, and patients initially present with comorbid injuries and are often unconscious or unresponsive for considerable periods immediately following the insult, which allows only subjective assessments. Initial assessments, then, can often be misleading or simply erroneous if they can be obtained at all after accounting for limitations in sensation and voluntary behavior. Accordingly, the nature of optimal outcomes for clinical trials investigating therapies for SCI remains a controversial and uncertain issue.7 Researchers and clinicians often disagree about what types of behaviors are important, or at least which are most relevant for analytical focus. SCI includes a complicated interplay of impairments, including neurological insult, autonomic dysfunction, changes in quality of life and community integration, pain, spasticity, and sensorimotor deficits, which do not necessarily recover along the same trajectories.2,6,11 Ideally, individual outcomes that have been standardized and have demonstrated statistical efficacy would be assessed at cellular, physiological, and behavioral levels for all of these behaviors6,7,10; however, this ideal is constrained by the reality of confirming the usefulness of the suite of assessments and of the clinical impossibility of administering them all to patients.12 Another divisive issue surrounding the outcomes debate is the definition of clinically meaningful change.6,13 Most researchers agree that subtle neurological changes should not be underestimated because subclinical function may indicate where recovery is more feasible,14 but small effect sizes in highly variable subject pools lead to nonsignificant results and trial “failures.”15 Although there are accepted thresholds for clinically meaningful change in most of the widely used outcome measures for SCI, these blanketing guidelines add little to the field in terms of recognizing therapeutic benefit for all patients. For example, most researchers acknowledge that the Berg Balance Scale (BBS) has the capacity to demonstrate change over time and that increases of 5 to 7 points (out of a total of 56 points given for 14 different actions) represent clinically meaningful improvement.16 Tests of ambulation speed and endurance, like the ten meter walk test (10MWT) and the six minute walk test (6MWT), are also recognized for their capacity to detect change over time, as well as their profound floor and ceiling effects and their disregard for assistive devices.10 Ditunno et al. report that only 14/124 participants were actually able to complete the 6MWT due to the severity of their paralysis.16 Participants are deemed “slow” walkers if their speed is < 0.8 m/s, and individuals ambulating faster than this are often fully functional.17 Another suggestion is that neither of these tests is relevant to real life situations, nor do they reflect the individual’s true capacity. To summarize, agreeing upon an all-encompassing guideline for clinical relevance may be completely unnecessary, if not obstructive. Gains of even 0.01 m/s, or doing so with a less invasive assistive device, could be of great personal relevance to an individual who was not able to walk at all before, whereas gains > 0.5 m/s could mean little to individuals who can already walk faster than the “fully functional” 0.8 m/s. Clinical significance, then, is a concept that must be carefully considered, especially when considering groups of SCI patients with wide-ranging skill levels and functional capacities. Over all, researchers recognize that a battery of powerfully sensitive instruments, focusing on several domains of function, is most desirable. Standardizing excellent outcome measures will lead to decreases in necessary sample size, increased positive findings, and more accurate prediction of when and which candidates are likely to benefit from interventions, ultimately decreasing cost.5 Outcome measures that are commonly used to document deficit and recovery after SCI are as varied as the typical sequelae of impairments they attempt to analyze. In a recent review, Alexander outlines several categories of popular assessments for SCI, including neurological—assessing behavior to understand the severity and prognosis of the injury; neuroimaging using magnetic resonance imaging to access neurological information; sensorimotor function using a variety of techniques to document motor and sensory capacity; functional potential using self-report and objective tests to monitor overall activities of daily living; extremity function using generic tests of hand or foot function to assess motor and sensory impairment of complex tasks; ambulation tests to monitor speed, endurance, and functionality; autonomic function using general measures for blood pressure, heart rate, and orthostatic regulation; colon and rectal function, typically documenting either physiology or subjective reports of activity; lower urinarytract function, mainly urodynamics; sexual function; pain; spasticity, typically assessed with electromyography (EMG); depression, quality of life; and participation.6 Devices for many of these categories were initially developed for other disorders and, thus, are currently not standardized or statistically tested for use in the SCI population.6,18 Much of the SCI literature focuses on just three of these categories: neurological status, motor and sensory function, and functional capacity. Rather than outlining the shortcomings of this limited focus (e.g., many have noted that there are few outcome measures that assess autonomic function, especially sweating, temperature regulation, and respiration,6,8,12 despite the fact that autonomic dysfunction is related to a majority of the most common causes of death for individuals with SCI19), this chapter addresses shortcomings within this focus, demonstrating how currently available outcome measures used within the popular categories are insufficient (Table 45.1). As soon as possible (i.e., when the patient is stable and conscious), patients diagnosed with SCI are assessed on the American Spinal Injury Association’s (ASIA) Impairment Scale (AIS).5,6 The AIS is an index based on a clinical neurological examination from which several measures of neurological damage are generated (neurological, sensory, and motor injury level).20 Reliability and validity of the AIS have been shown time and again, but although it is accepted and used as the gold standard outcome measure for SCI, most researchers and clinicians acknowledge that the AIS has limitations, specifically in sensitivity.6,12 Two of the most obvious contributions to the insensitivity of the AIS are its exclusion of tests for thoracic motor function, muscles that are innervated by the thoracic segments where most of the limited recovery is anticipated to occur, and its classification of sensory function into “normal,” “absent,” or “abnormal” categories.12 Simply put, AIS is valid as a tool for classification of injuries but not as an outcome measure,21 and, accordingly, many of the other outcome measures we will discuss demonstrate validity by correlating with AIS classifications. A plethora of devices have been either developed for SCI or modified from use for other disorders to assess either functional, motor, or sensory impairments, or some combination of the three. These outcome measures are typically considered either functional (i.e., they monitor an individual’s capacity to perform various tasks) or electrophysiological (referring mostly to the method by which neurological functioning is assessed). Probably the most commonly used outcome measure for neurological disorders worldwide, the Functional Independence Measure (FIM) monitors functional ability in daily activities, focusing on the amount of assistance, or burden of care, required.5,10,13 Although the FIM requires extensive training to administer correctly, research has shown that the test is reliable and has good predictive validity, especially for life satisfaction and burden of care.13 The FIM was not originally developed for SCI, and although this allows the score to include all levels of disability, its broadness creates several issues for use in SCI.10,13 First, some SCI patients simply cannot perform FIM tasks within 72 hours of their injury, due to the unstable nature of their condition, lack of consciousness, or other injuries.13 Most importantly, the FIM suffers from profound insensitivity to impairments in individuals with less severe SCI who do not require assistance.10 In short, the FIM simply has no real utility for SCI recovery assessment.13 After encountering the aforementioned issues with the FIM, researchers specifically designed the Spinal Cord Independence Measure (SCIM) to comprehensively test functionality in SCI.6,13 The SCIM is made up of three subscales that test individuals with SCI on their ability to perform relevant daily tasks and their corresponding assistance needs.5,13 The tool is growing in popularity and usage, especially as a complement to AIS assessments, and is more sensitive than both the AIS and the FIM, particularly for mobility, bladder, bowel, and respiratory functioning.13 Psychometric analyses demonstrate that the SCIM is valid and reliable and has good predictive validity, and can, therefore, be used to plan rehabilitation programs.13 Still, even as the SCIM is much more sensitive than other measures of global disability, it also fails to detect either subclinical changes, improvements on either end of the disability spectrum, or nuances of motor behavior, such as quality of gait, balance, and real-life mobility (like ambulation over uneven surfaces).13 Table 45.1 Advantages and Limitations of Commonly Used Outcomes Assessments
Electrophysiological Predictors of Lower Limb Motor Recovery: The Rehabilitation Perspective
 Translation of Spinal Cord Injury Research
Translation of Spinal Cord Injury Research
Outcome Measures
 Commonly Used Outcome Measures for Spinal Cord Injury
Commonly Used Outcome Measures for Spinal Cord Injury
Clinical Neurological Assessment
Functional, Motor, and Sensory Assessments
Functional Assessment of Neurological Functioning
Functional Independence Measure
Spinal Cord Independence Measure
Outcomes assessments: electrophysiological measures | Advantages | Limitations |
Reflex testing | • Statistically valid and reliable • Sensitive and applicable for individuals with SCI at all levels and severities • Provides objective, quantitative data • Correlated with spasticity • Indicative of both short- and long-term plasticity in the nervous system | • Assessment requires time, training, and equipment • Need standardization of protocol and data management • Data can be affected by extraneous factors that produce noise that can lead to misinterpretation |
Sensory evoked potential | • Statistically valid and reliable • Sensitive and applicable for individuals with SCI at all levels and severities, conscious patient involvement is limited • Provides objective, quantitative data | • Assessment requires time, training, and equipment • Need standardization of protocol and data management • Assessment is restricted to fast conducting tracts • Monitors specific pathways only |
Motor evoked potential | • Statistically valid and reliable • Sensitive and applicable for individuals with SCI at all levels and severities, conscious patient involvement is limited • Provides objective, quantitative data | • Assessment requires time, training, and equipment • Need standardization of protocol and data management • Assessment is restricted to fast conducting tracts • Monitors specific pathways only |
Electrical perceptual threshold (EPT) | • Statistically valid and reliable • Sensitive and applicable for individuals with SCI at all levels and severities • Provides objective, quantitative data • Assessment of both fast and slow conducting tracts • Can be integrated to provide a complete sensorimotor analysis | • Assessment requires time, training, and equipment • Need standardization of protocol and data management • Conscious patient involvement is required |
Brain motor control assessment (BMCA) | • Statistically valid and reliable • Sensitive and applicable for individuals with SCI at all levels and severities • Provides objective, quantitative data • Assessment of both fast and slow conducting tracts • Can be integrated to provide a complete sensorimotor analysis | • Assessment requires time, training, and equipment • Need standardization of protocol and data management • Conscious patient involvement is required |
Walking Index for Spinal Cord Injury
The Walking Index for SCI (WISCI) is an outcome measure that evaluates walking in a standard environment while accounting for bracing, ambulation aids, and assistance.5,10 The score depends on the participant’s device requirements and is therefore widely inclusive, but it does not account for speed, gait quality, or sit-to-stand capacity.10 Psychometric analyses have shown the test’s reliability and validity by correlating scores with the lower extremity motor score of the AIS, the FIM, and measures of balance, speed, and distance.6,10,16 Once again, however, this outcome measure lacks sensitivity at both ends of the disability spectrum, in chronic injuries, and for subclinical changes.10
Ten Meter and Six Minute Walk Tests
Outcome measures that assess walking speed over a given distance or within a specified time frame are considered overall measures of walking ability and provide some predictive value for independent ambulation.10,17 The 10MW and 6MW tests were originally developed and standardized to detect functional capacity in other neurological disorders because it is assumed that gait mechanics, strength, and proprioception all have a direct effect on ambulation speed.10,22 Psychometric analyses have shown that both timed measures are more reliable, valid, and sensitive than qualitative functional measures, at least in individuals with incomplete SCI who already have the capacity to ambulate.10,23 Both tests produce substantial floor and ceiling effects, given that some individuals are unable to perform the tests at all and others can perform them without any real effort.10 Additional methodological issues include decreased reliability with repeated testing, environmental effects (like the number of turns on a course), examiner effects (such as type and amount of encouragement given),10 and use of compensation, such as assistive devices.
Electrophysiological Assessment of Neurological Functioning
Another method for assessing outcomes after SCI includes electrophysiological measures (see Chapter 38 for a thorough review). These noninvasive and precise tests have the potential to fill the field’s need for a tool that can predict outcome early after injury.8,24–27 Sensory and motor evoked potentials and reflex testing can provide information beyond the clinical exam, be completed in impaired patients, help more completely characterize the injury, and detect changes, allowing prompt responses in treatment plans.8,26,28 Electrophysiological assessments offer the potential to detect subclinical changes in neurological functioning because they are objective and much more sensitive than the AIS or functional tests.6,8,12,14,15,27,29–31 The tests are sensitive not only for monitoring recovery of motor and sensory capacity as well as spasticity but also for establishing baseline measures of impairment.32,33 One study reported that an electrophysiological assessment of sensory capacity placed 41% of subjects into less severe categories of injury than suggested by their AIS scores.31 Therefore, most researchers agree that electrophysiological tests will increase the clinician’s ability to determine the level and “completeness” of an SCI in addition to monitoring outcomes and suggest administering them alongside the AIS to complement and enhance evaluations.8,15 Psychometric analyses have shown that, in general, they are sensitive, reliable, and valid because they closely correspond to actual neurological functioning,29,33 and over all these measures are quickly becoming viewed as the best tools for detecting functional capacity of neurons after injury.6,30,31
The rationale behind electrophysiological testing is that retention or recovery of voluntary control or reflexive activation of a muscle or conscious perception of a sensation is indicative of intact, uninjured, or recovered neurological processes. Sensory evoked potentials demonstrate the integrity of sensory impulse transmission, are not affected by spinal shock, and can be conducted in unconscious patients, whereas motor evoked potentials demonstrate the integrity of motoneuron pathways, can be conducted painlessly in conscious patients, and can be used to supplement the clinical exam in characterizing the injury and assessing its extent.8,26 Peripheral testing, such as EMGs and reflex assessments, can be used to distinguish peripheral lesions, assess motoneuron functioning, and detect the development of neuropathologies, such as spasticity.8,26,34 There are many different protocols for electrophysiological tests, but they all share the same basic principles. An electrical stimulation is given to a sensory dermatome (usually mimicking key AIS evaluation points) or muscle, and the pattern of output response is noted either by patient report or EMG.27,31 Decreased latency of response indicates improved neurological conduction and, accordingly, some degree of neurological recovery.12,35,36 These measures have already been successfully used to demonstrate improved conduction following pharmaceutical intervention,36 the reestablishment of synaptic activity after transplant therapy (despite the failure of these grafts to regenerate),37,38 and a distinction between the capacity for patients with sexual impairments to achieve or maintain erections.39 Some studies also tout the prognostic value of these assessments, especially for sympathetic failure and autonomic hyperreflexia,8,39 bladder functioning, and future ambulatory capacity.8
Electrical Perceptual Threshold
A method for quantitative sensory testing, the EPT test, was developed by Belci et al.40 for the assessment of cutaneous sensibility and has been validated against the AIS12 (see Chapter 39 for a thorough review). The EPT has also demonstrated repeatability for both inter- and intrarater trials.29 In brief, the EPT measures the threshold, or minimally detectable sensory appreciation, of constant current square wave pulses (0.5 msec) applied at a frequency of 3 Hz to an adhesive, disposable cathodal electrode applied to the AIS test point of a dermatome. A larger indifferent anode is attached to the skin at a remote location. The method of limits is then used to determine threshold, increasing the strength of stimulation until a participant just reports sensation at the location of the cathode, and subsequently reducing it until sensation is reported as lost. The test is repeated three times for each dermatome, and the average of the descending thresholds is taken as the EPT. The method is well tolerated by individuals and can be performed in less time than the AIS sensory exam, with relatively modest increases in total cost.
The technique of perceptual threshold to cutaneous electrical stimulation (EPT) was developed on persons with intact neurological systems. Thresholds differed according to the dermatome tested (n = 7 C3-L5), but a correlation was established for equivalent dermatomes on the left and right sides. Women had slightly lower EPTs than men, but the difference was significant only for the lumbar (L3, L5) dermatomes and not elsewhere (C3, C4, C5, C6, and T8). The technique has been adapted to assess individuals with SCI at any level and of any impairment grade.31 First, a thorough evaluation of normal EPT values was established for 30 control subjects for all dermatomes (C3 through S2). The results confirmed and extended the earlier study with regard to variation in EPT between dermatomes and the good correlation between left and right sides. The data provided a template against which to evaluate subjects with SCI. EPT was measured in 45 patients at AIS key sensory points for selected dermatomes at, above, and below the clinically defined level of lesion. The level of lesion determined by AIS was compared with the EPT readings and was the same in 48% of tests. In 41%, the EPT was actually more cranial than the clinical level, and in only 11% of tests was it lower. The EPT also revealed asymmetries in sensory perception that were not evident from the AIS grading, particularly in the zone of partial preservation. For dermatomes with preserved but impaired light touch (AIS grade 1) and absent pinprick sensation (AIS grade 0), EPT was measurable but elevated above control values. This provided preliminary evidence that EPT reflects conduction in the posterior column pathways rather than the anterolateral spinothalamic tract. A more recent unpublished study has produced further evidence to support this association (Fig. 45.1). The conclusion was that EPT provides a simple, reproducible quantitative sensory assessment of the level and degree of impairment in SCI. EPT adds to the sensitivity and resolution of the AIS clinical grading by (1) increasing objectivity through machine-rather than human-applied stimulation; (2) providing a continuous numerical scale of sensitivity, rather than a discontinuous ordinal scale, that is able to reveal asymmetries (left, right) and subtle changes with time/recovery not indicated by AIS testing; and (3) revealing deficits in sensory perception not detected by AIS sensory ratings (i.e., raised EPT for AIS light touch and pinprick scores of 2 (normal).29
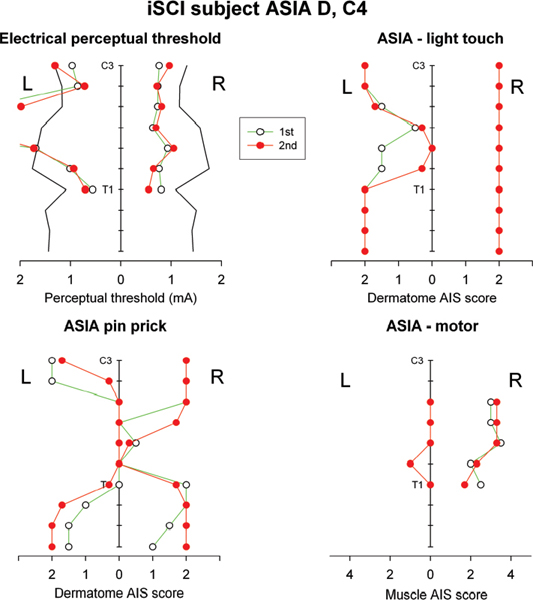
Fig. 45.1 Results of electrical perceptual threshold (EPT) and American Spinal Injury Association (ASIA) Impairment Scale testing on an individual with an ASIA D spinal cord injury at C4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


