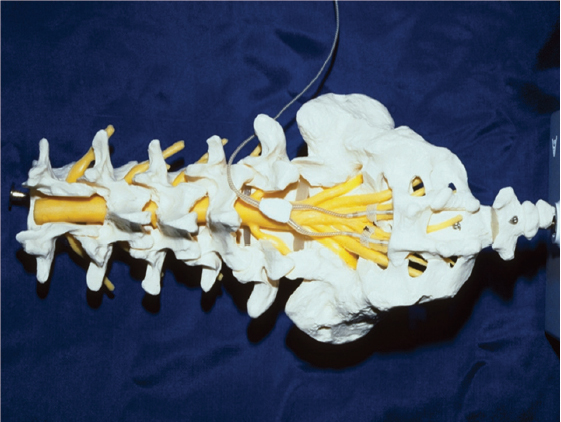
47 Key Points 1. After spinal cord injury, surviving peripheral nerves can be activated safely for many years by electrical stimulation. 2. Stimulation of efferent nerves can produce muscle contraction and useful function of the diaphragm, bladder, bowel, and limbs, and seminal emission. 3. Stimulation of afferent nerves can modify reflex activity and pain. 4. Stimulation of muscles via the skin surface may be useful for exercise. 5. Implanted devices, like pacemakers, facilitate long-term restoration of function. After injury to the spinal cord, the remaining intact nerves in the central or peripheral nervous system can be stimulated electrically for various purposes. These may be diagnostic, to estimate the extent of damage to upper or lower motor neurons. Therapeutic purposes may include providing exercise or modifying existing function by inhibiting or enhancing reflexes or inhibiting pain, a process sometimes known as neuromodulation.1 It is also possible to restore some useful movement to paralyzed muscles by stimulation of their lower motor neurons, using devices known as neural prostheses, to provide functional electrical stimulation (FES).2 Muscles with intact lower motor neurons may undergo disuse atrophy but remain responsive to electrical stimulation applied directly to the muscle cell or to the motor nerve. Such stimulation can be effective many years after spinal cord injury (SCI) in producing contraction, reversing disuse atrophy, and restoring function. Denervated muscles undergo progressive atrophy that can probably not be prevented by electrical stimulation. The main requirements have been to develop safe and effective patterns of stimulation that can be applied long term using commercially viable devices. These requirements have been met in several areas. The least invasive techniques of applying electrical stimulation use electrodes applied to the skin surface. These are suitable for diagnostic and therapeutic purposes but are not always sufficiently selective or reproducible for restoring useful function; managing the electrodes, wires, and stimulators can become inconvenient for long-term use. Fine wire electrodes inserted with a hypodermic needle are widely used for diagnostic purposes, and some, such as percutaneous electrodes, can be left in place safely for months or years for research purposes. They allow specific reproducible stimulation patterns to be developed and evaluated using stimulators outside the body, although some care is needed for maintaining the skin exit sites and external leads. For long-term functional use, it is most convenient to have stimulators fully implanted within the body. These may be powered by implanted batteries, in which case revision surgery is required every few years to replace the batteries, although implantable rechargeable batteries are being developed. Alternatively, the implant is powered by radio frequency transmission from a controller outside the body; such a controller can be reprogrammed or replaced conveniently. All implanted components must be designed to cause no harm to the tissues, whether electrical, chemical, or mechanical, and to be resistant to damage by body fluids or movement. Ideally, the implant should have a lifetime longer than that of the user, but in case of faults or improvements, provision should be made for repair or replacement. Electrical stimulation can increase strength in paralyzed lower-limb muscles of people with SCI;3 with prolonged aerobic exercise, it can also improve muscle endurance.4,5 There are also cardiovascular benefits,6 increased HDL level,7 and improved insulin sensitivity.8 Combining arm cycle ergometry (ACE) and electrical stimulation leg cycle ergometry (LCE)9,10 may have enhanced cardiovascular benefits. Chronic electrical stimulation LCE may increase lower extremity bone mineral density, or at least slow the progression of lower extremity osteopenia,11–13 but when FES training is discontinued, lower extremity bone mineral density reverts to its previous level. There are variable reports of the effects on spasticity of electrical stimulation of the limbs, posterior columns, or sacral nerves,14–17 and this is not widely used for spasticity control after SCI. Neuromodulation shows some promise for inhibition of the hyperreflexic bladder by stimulation of the dorsal genital nerves or the pudendal nerves, particularly if it can be applied specifically when a bladder contraction is detected.18 Electrical stimulation is sometimes effective for neuromodulation of neuropathic or nociceptive pain after SCI, a subject addressed in Chapter 17. In individuals with high tetraplegia but preserved bilateral phrenic nerve function, the phrenic nerves can be stimulated electrically to induce diaphragm contraction so that the patients no longer require mechanical ventilation. In conventional methods, the diaphragm is activated by electrodes implanted surgically on both phrenic nerves in the thorax and connected to an implantable receiver with an external power supply and transmitter. Such systems have been implanted in over a thousand patients over the last 3 decades, and there are now three commercially available devices.19 This method requires a thoracotomy, in-patient hospital stay, and high cost. In addition, the procedure requires phrenic nerve dissection and placement of electrodes directly on the nerves and therefore carries some risk of phrenic nerve injury. These disadvantages have limited the number of individuals willing to undergo diaphragm pacing. An alternative procedure, currently under investigation, involves placement of electrodes into the muscular portion of the diaphragm near the entrance points of the phrenic nerve into the diaphragm.20 This procedure is performed laparoscopically and can be done on an out-patient basis. The advantages of intramuscular placement of phrenic nerve electrodes include less risk of phrenic nerve injury and a reduction in overall cost. When placed near the motor point, as determined by intraoperative testing, intramuscular diaphragm stimulation results in virtually the same inspired volume production as that resulting from direct phrenic nerve stimulation. Forced expiration and cough production involve lower intercostal and abdominal muscle activation. Electrical stimulation of abdominal muscles using electrodes on the skin has been found to produce cough comparable with that produced with the manual assistance of a therapist.21 Electrical stimulation of the expiratory muscles has also been investigated with electrodes implanted on the lower thoracic spinal cord.22 In patients with preserved conus medullaris and sacral nerves, improved voiding, continence, and erection can be achieved by electrical stimulation of the sacral nerves or roots. An implantable stimulator capable of improving bladder, bowel, and erectile function has been available commercially in some countries since 1982 and has been implanted successfully in several thousand patients with SCI (Fig. 47.1). Electrical stimulation of the sacral motor roots causes contraction of the bladder and lower bowel but also contraction of the external sphincters, which might be expected to be counterproductive and even harmful. However, intermittent bursts of stimulation can produce sustained contraction of the smooth muscle of the bladder or bowel while allowing the sphincter to relax during the intervals and permit urine or stool to be passed safely. Urine is voided with low residual volumes, resulting in a significant reduction of urinary tract infection.23,24 Fig. 47.1 Location of surgically implanted extradural electrodes on S3 and S4 nerves bilaterally, and one potential location for intradural posterior sacral rhizotomy at the level of L1. Reflex incontinence can be reduced dramatically by surgical division of the sacral sensory roots, which is often performed when a sacral anterior nerve root stimulator is implanted, and this also reduces the risk of renal damage and autonomic dysreflexia. However, it also abolishes desirable functions, such as reflex erection and reflex ejaculation, and although there are alternative means of restoring these, its benefits must be weighed against its disadvantages. Restoration of voiding and continence by the foregoing techniques reduces the need for antibiotic and anticholinergic medication, appliances, and hospital visits, resulting in reduced annual costs for bladder and bowel care; studies in Europe and the United States have indicated that within 5 to 8 years, these savings cover the cost of implanting and using the device.25,26 Current research is addressing alternatives to surgical rhizotomy for reducing hyperreflexia of the detrusor and sphincters, such as electrical block and inhibition of muscle contraction. Hyperreflexic contractions of the bladder can be inhibited by electrical stimulation of sacral afferent nerves by a variety of routes, including electrodes on sacral dermatomes, in the anus and vagina, in the ventral sacral foramina, on the dorsal genital nerves, and even on the posterior tibial nerves, but these techniques have not proved sufficiently effective in reducing reflex incontinence after SCI to be accepted into widespread clinical use. Stimulation of intact parasympathetic efferent axons in S2 can produce erection for as long as stimulation is maintained. Electrodes are usually implanted at the same time as electrodes for restoring bladder and bowel function as already described.27 Seminal emission can be obtained in most patients after SCI by applying stimulation via an electrode inserted temporarily into the rectum for “electroejaculation.”28 Restoration of useful function to patients with tetraplegia and preserved lower motor neurons to some upper limb muscles can be achieved by electrodes implanted in or on muscles in the forearm and hand and connected to an implanted stimulator. Implanted systems are usually enhanced by tendon transfers. Conversely, stimulation of paralyzed but innervated muscles can increase the number of potential tendon transfers. Stimulation of flexors and extensors of the fingers and thumb, the adductor and opponens pollicis muscles, and sometimes wrist balancing muscles, can produce palmar grasp and release for large objects and key-pinch grasp for smaller items. Tendon transfers are used to stabilize the thumb, synchronize finger movement, and compensate for some denervated muscle groups. A first-generation implant of this type using eight electrodes was implanted in over 200 patients with C5 or C6 tetraplegia by 35 centers in at least eight countries.29 A user satisfaction survey at least 6 months after completing training with the device confirmed that users needed less adaptive equipment and less assistance from others; on average they used the device 5 days a week, and nearly half used it every day. The majority of patients were very satisfied with the system, and 80% said they would recommend it to others.30 Second-generation devices with 12 active stimulation channels and two myoelectric signal sensing channels are being investigated for improved control of the intrinsic muscles of the hand, wrist balance, pronation and supination, and elbow and shoulder position.31 The triceps muscle is controlled to produce elbow extension and allow reaching above the head. Research is also in progress on control of the paralyzed shoulder for people with high tetraplegia. Such control is complex because of its many potential movements, and there are limited voluntary movements available to a high tetraplegic to control a stimulator. Direct electrical connections with the cerebral cortex are being investigated as a control interface.32 Electrical stimulation can provide individuals with thoracic or low cervical injuries with the ability to exercise, stand, transfer, and perform simple stepping. Standing can be achieved by activating both quadriceps, whereas a stride can be produced by activating the quadriceps of one leg while initiating a flexion withdrawal in the other. Selected individuals with neurologically incomplete SCI and some preserved motor and sensory function have been able to regain household or community mobility.33 Hybrid systems combining stimulation with orthoses have been fitted to patients with complete or incomplete thoracic or low-level cervical injuries. The energy required to operate these hybrid systems is less than that required for braces alone, but it increases rapidly with walking velocity due to an increased reliance on the arms and trunk muscles to move the body forward.34 For long-term clinical application, implanted systems offer major advantages over surface and percutaneous stimulation, including improved convenience, cosmesis, and reliability. Exercise and standing have been reported with a cochlear implant modified to deliver 22 channels of stimulation,35 and a 12-channel system for activation of the L2–S2 motor roots has been applied to a handful of volunteers.36 An eight-channel implanted stimulator with electrodes implanted in or on muscles has undergone clinical testing in a small number of subjects.37 On average, subjects were able to stand for more than 10 minutes with 85% of their body weight on their legs. Some subjects were able to achieve short-distance mobility with a swing-to gait. Reciprocal stepping can be achieved with 16 channels of stimulation through the addition of a second implant to activate hip flexors and ankle dorsiflexors.38 All standing and walking neuroprostheses require assistive devices, such as crutches, walkers, or additional bracing, and this restricts the activities and environments in which the neuroprosthesis can be used. Current experimental lower extremity FES systems facilitate brief mobility-related tasks, allowing people with paraplegia to overcome physical obstacles, negotiate architectural barriers, and reach and manipulate objects that are otherwise inaccessible from the wheelchair. Electrically stimulated exercise of the lower limbs has been shown to enhance new neural cell birth and survival following chronic SCI in rats.39 This demonstrates that FES can enhance cellular regeneration in the injured adult central nervous system and raises the possibility of controlled electrical activation of the nervous system for optimizing spontaneous regeneration and functional recovery in neurological injuries. The trend in surgery to less invasive techniques affects the design and implantation of neural prostheses. Many electrodes and cables can be implanted percutaneously and tunneled under the skin to connect to a stimulator placed subcutaneously like a pacemaker. Other developments include standards that will allow many components to be interconnected in wired and wireless networks within the body. This will allow systems to be assembled and upgraded as needed with minimal surgery; it also allows compatible components to be made by different manufacturers, permitting larger-scale production and reduced costs and increasing the flexibility with which systems can be assembled. This is likely to improve further the balance between function restored and the investment of time, energy, and money required. Ultimately, the provision of improved care to SCI patients depends not primarily on development of new techniques but on their delivery by clinicians and funding agencies. This depends on collaboration to translate research into clinical application and on recognition by those responsible for health care funding of the return on investment achieved by restoring function to people with damage to the central nervous system. Pearls
Electrical Stimulation Following Spinal Cord Injury
 Techniques
Techniques
 Therapeutic Effects
Therapeutic Effects
Exercise
Neuromodulation
 Restoration of Function
Restoration of Function
Respiratory Function
Inspiratory Muscle Pacing
Expiratory Muscles and Coughing
Bladder, Bowel, and Sexual Function
Voiding
Continence
Erection
Ejaculation
Upper Limb Function
Lower Limb Function
Surface and Percutaneous Electrode Systems
Fully Implanted Electrode Systems
 Future Trends Clinical
Future Trends Clinical
Technical
 Bladder, bowel, and sexual function after spinal cord injury can be substantially improved by an implanted stimulator of the sacral nerves or nerve roots.
Bladder, bowel, and sexual function after spinal cord injury can be substantially improved by an implanted stimulator of the sacral nerves or nerve roots.
 Hand grasp can be partly restored to tetraplegic subjects by electrical stimulation of peripheral nerves and muscles using an implant.
Hand grasp can be partly restored to tetraplegic subjects by electrical stimulation of peripheral nerves and muscles using an implant.
 Cough can be improved significantly in tetraplegic and high paraplegic patients by stimulation of the expiratory muscles, thereby reducing respiratory complications.
Cough can be improved significantly in tetraplegic and high paraplegic patients by stimulation of the expiratory muscles, thereby reducing respiratory complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


