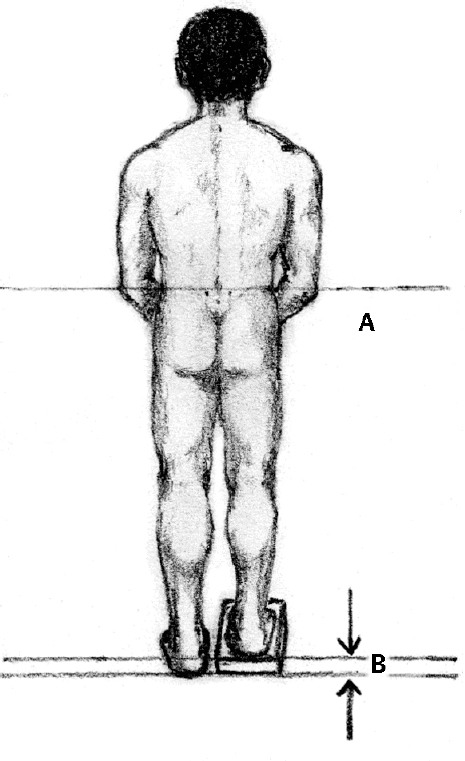
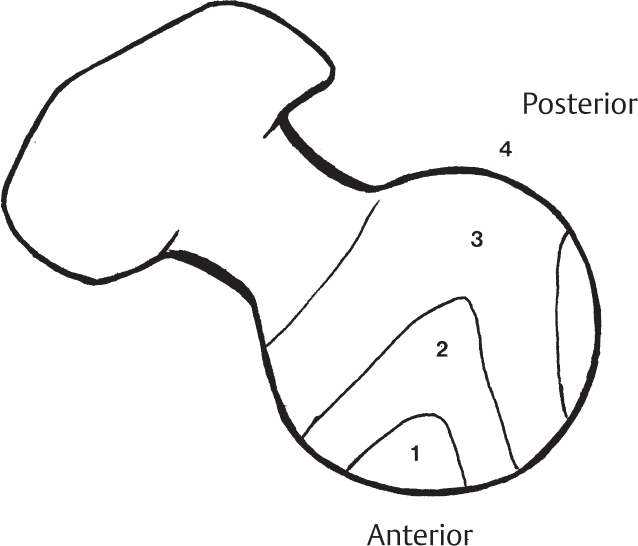
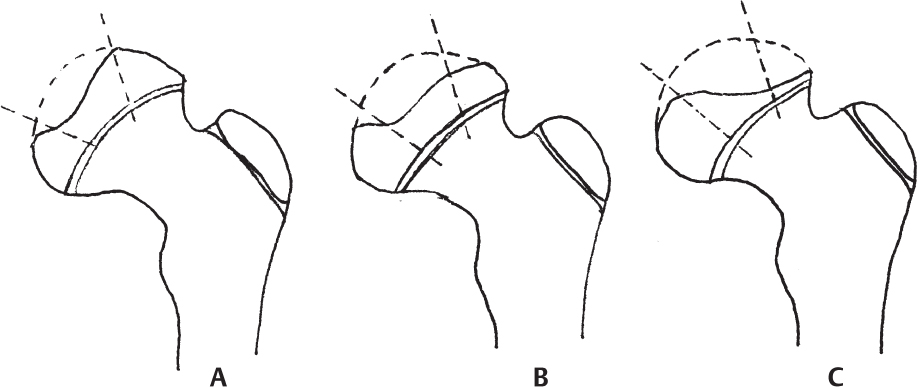
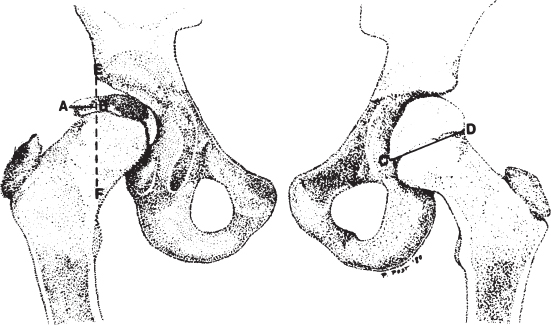
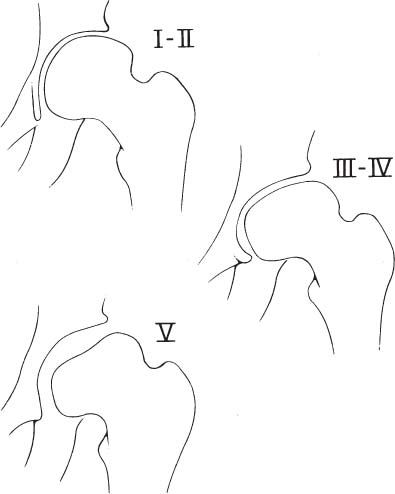
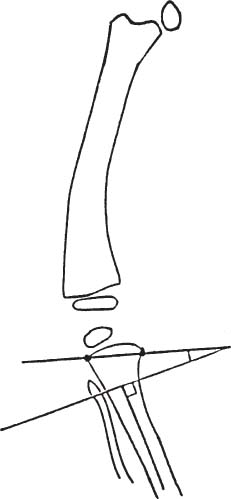
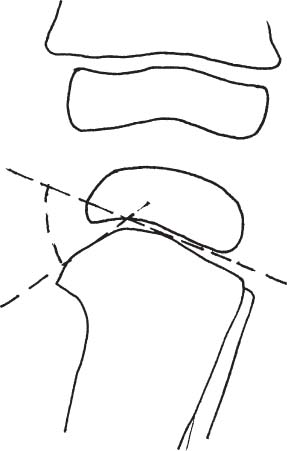
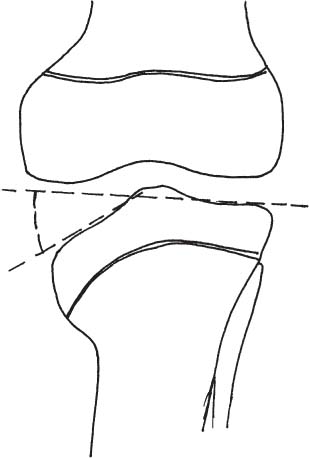
2 Disorders of Skeletal Growth and Development Selected pediatric orthopedic conditions are summarized here with emphasis on central concepts and parameters. The goal is to provide a working knowledge for treatment. Principles and specific treatment details are covered more extensively in standard texts. Other major topics are covered in Chapters 3 (genetic syndromes), 4 (neuromuscular disorders), and 5 (trauma). 1. Inequalities of lower-limb length of up to 1 to 1.5 cm are within normal variation of the population. 2. Inequalities of less than 2.5 cm do not cause back pain or noticeable limp. 3. Length inequalities are a much less common cause of limp than are joint disorders (contracture, pain) or muscle weakness. 4. The gait disturbance caused by inequality of limb length is usually subtle and consists of pelvic drop and compensatory flexion of the knee of the long limb and equinus of the ankle of the short limb. 5. Most congenital inequalities of limb length behave in a proportionate fashion with growth; that is, the ratio of the short leg to the long leg is constant throughout growth. 1. Congenital short femur, proximal focal femoral deficiency, tibial or fibular hemimelia, hemiatrophy, hemihypertrophy. Multiplier method predicts final discrepancy (see Chapter 1). 2. Systemic disorders: Ollier disease, fibrous dysplasia, osteochondromatosis, osteogenesis imperfecta, cerebral palsy. 1. Developmental dysplasia of the hip (DDH) (length difference resulting from growth disturbance or osteotomy). 2. Legg–Calve–Perthes disease (may be up to 4 cm if proximal femoral phy-seal growth slows early. 3. Blount disease 4. Trauma (fracture overlap, growth arrest) 5. Osteomyelitis with physeal arrest 6. Foot deformities 1. Block method: Palpate height of iliac crests in standing patient. Add height to short limb by measured blocks until equal. This is a good screening method to determine whether further, more precise measurements are needed. Although not as precise as radiographic measurements, it does take into account all factors such as contracture and shortening occurring within the foot or pelvis (Fig. 2.1). 2. Tape method: Measure from inferior margin of the anterior superior iliac spine (ASIS) to the medial malleolus. This method can be inaccurate in overweight patients or in those who have had anterior hip surgery and does not include foot discrepancies. 3. Scanogram: Images of both hips, knees, and ankles are taken in one position alongside a radiographic ruler. This method does not account for foot discrepancies or contracture. 4. Computed radiograph: Computes lengths and angles of segments. 1. If discrepancy is less than 2.5 cm in a mature person, no treatment is needed. 2. 2.5 to ~4 cm: Leg lift or epiphyseodesis or shortening of corresponding long segment (see section 1). Fig. 2.1 The standing block test. A block is added under the short limb (B) until the pelvis is palpated to be level (A). Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A (10):1432–1446 Sabharwal S, Zhao C, McKeon JJ, McClemens E, Edgar M, Behrens F. Computed radiographic measurement of limb-length discrepancy: full-length standing anteroposterior radiograph compared with scanogram. J Bone Joint Surg Am. 2006; 88(10):2243–2251 Developmental dysplasia of the hip (DDH) is caused by forces acting on the hip in utero. The risk is increased by abnormalities of connective tissue. DDH is a spectrum, from hips that are subluxatable to dislocatable (Barlow positive) to dislocated (Ortolani positive). The combined incidence of these groups is 2 to 5 per 1000. All hips should be screened by a knowledgeable examiner at birth and again within the first few months of life. The infant should be made as quiet and comfortable as possible for the examination, using warmth, contact, low light, feeding, or a pacifier. Abnormal physical signs marked with an asterisk (*) should prompt reexamination or ultrasound, with treatment if these are abnormal. The following factors increase the risk of hip dysplasia in descending order and should prompt reexamination or ultrasound: 1. Positive family history of DDH 2. Breech position at the end of gestation (5% are unstable). A breech female should have a screening ultrasound. 3. Large birth weight 1. Appearance at rest: The affected side is more adducted at rest in unilateral cases and may have a deeper or extra high fold proximally. *2. Asymmetric passive abduction: Dislocated hip will lack passive abduction compared with the normal side (Fig. 2.2A). *3. The Barlow test will cause pistoning of the proximal femur if dislocatable (Fig. 2.2B). Fig. 2.2 (A) Asymmetric abduction; left side is dysplastic. (B) Barlow test (done on one hip at a time). (C) Ortolani test. *4. The Ortolani test will cause a “clunk” as a dislocated hip is relocated. Examine each hip separately; stabilize the pelvis with the other hand (Fig. 2.2C). Note that the Ortolani and Barlow tests are for translation of the femur. A “click” per se is not a positive test; only 1% of patients with a click have dysplasia. A click may come from the patella or the meniscus of the knee as well as from the fascia lata or a synovial fold in the hip. 6. Significant foot deformity or torticollis may increase the risk of hip dysplasia and should prompt a careful examination of the hips. In the older child with hip dysplasia, the signs progressively change. Reducibility of the hip in the awake patient is lost after about 3 months, and one must rely more on indirect signs: 1. Asymmetric passive abduction 2. Galeazzi test will show thigh shortening on the side that is dislocated (Fig. 2.3A). The pelvis should be kept level during this test. 3. Leg-length discrepancy 4. Trendelenburg gait 5. Palpable femoral head posterior to the acetabulum 6. Nélaton line (an imaginary line between the ASIS and the ischium) should lie superior to the trochanter (Fig. 2.3B). 7. Klisic line between the greater trochanter and the ASIS should project cephalad to the umbilicus (Fig. 2.3C). 8. Increased lumbar lordosis (if bilateral) is due to posterior displacement and mechanical disadvantage of hip abductors. The role of ultrasound in diagnosis of dysplasia varies regionally. Its benefit is its ability to show cartilage and other soft tissues, as well as observe stability in response to stress. Interpretation of an ultrasound considers both static and dynamic findings. The ultrasound view is named according to the direction of the transducer—transverse or coronal—and the position of the hip—neutral or flexion. The highest frequency possible (3 to 7 MHz) will give the best resolution, but this must usually be reduced with age to obtain adequate penetration. Fig. 2.3 (A) Galeazzi sign shows apparent thigh shortening on dysplastic side (right). (B) If dislocated, the greater trochanter will lie proximal to Nélaton line (anterior superior iliac spine to ischium). (C) Klisic line in the normal hip falls above the umbilicus. In this view, the landmarks are similar to those seen on a plain radiograph, when the transducer is in the mid acetabular plane. 1. The stability and gross appearance are the most important features. 2. The following other parameters may be checked (Fig. 2.4): Fig. 2.4 (A) Ultrasound, coronal view. FhC, femoral head coverage. α angle, acetabular roof line. β angle, slope of labrum. (B) Transverse view. Note the “U” formed by the metaphysis and the acetabulum. a. Femoral head coverage: The percent of the femoral head medial to the outer line of the ilium. This should be greater than 50%. b. Alpha angle, or acetabular roof line, between the lateral ilium and the bony acetabular roof. It is analogous to the acetabular index and should be greater than 60 degrees. c. Beta angle, or slope of the labrum versus the lateral wall of the ilium. It should be less than 55 degrees, indicating a downward slope of the labrum. In this view the hip is flexed and the transducer is placed posterolaterally in the transverse plane of the body. The combination of echoes from the femoral metaphysis and the acetabulum normally form a “U.” When dislocated, the femoral head comes to lie lateral and posterior to the acetabulum, and the U is disrupted. 1. Grades of hip dislocation according to Tonnis indicate the position of the ossific nucleus relative to Perkin vertical line (p) and Hilgenreiner horizontal line (h) (Fig. 2.5). a. Nucleus medial to Perkin line b. Nucleus lateral to Perkin line c. Nucleus at Hilgenreiner line d. Nucleus above Hilgenreiner line 2. The acetabular index is the angle formed between the Hilgenreiner line and the inner and outer borders of the acetabular roof (Fig. 2.6A, right hip). It is useful in assessing hip development in early years, before the center of the femoral head can be accurately identified. The normal values are shown in Fig. 2.6B. 3. Center edge angle of Wiberg (CEA) a. CEA measures the coverage of the femoral head by the acetabulum (Fig. 2.6A, left hip). Long-term follow-up studies by Wiberg have shown a correlation between development of symptoms after maturity and CEA below 20 degrees. Fig. 2.5 Tonnis grades of hip dislocation (p = Perkins? line and h = Hilgenreiner? s line). Fig. 2.6 (A) Acetabular index measurement (right hip) and measurement of center edge angle of Wiberg (left hip). (B) Acetabular index normal values for age. (Part B from Tonnis D. Normal values of the hip joint for the evaluation of x-rays in children and adults. Clin Orthop Relat Res. 1976;119:41 (Fig. 2). R eprinted with permission.) • Lower limit of normal • 5 to 8 years: 19 degrees • 9 to 12 years: 25 degrees • 13: 26 degrees • Less precise under 5 years Tönnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res. 1976; (119):39–47 The following is a general algorithm for management of dysplasia (Fig. 2.7). Guidelines given may be modified based upon individual factors. Fig. 2.7 General algorithm for management of pediatric hip dysplasia. Grissom L, Harcke HT, Thacker M. Imaging in the surgical management of developmental dislocation of the hip. Clin Orthop Relat Res. 2008;466(4):791–801 Guille JT, Pizzutillo PD, MacEwen GD. Development dysplasia of the hip from birth to six months. J Am Acad Orthop Surg. 2000;8(4):232–242 Legg–Calve–Perthes disease (idiopathic avascular necrosis [AVN] of the immature femoral head) is most commonly seen in children aged 4 to 10 years. Five percent of patients develop bilateral involvement, but this is almost always at different times (asynchronous). Synchronous involvement should suggest the possibility of skeletal dysplasia, hypothyroidism, or steroid use. 1. Minimal or no history of trauma history 2. Stiffness 3. Intermittent mild pain or no pain at all 1. Mild Trendelenburg gait 2. No pain with gentle motion 3. Limitation of extremes of motion, especially abduction and internal rotation 1. Chronological sequence a. Initially may appear normal b. Failure of nucleus to grow versus opposite side c. Subchondral “crescent” sign best seen on lateral view, present in one third of cases d. Fragmentation of nucleus with resorption e. Epiphyseal extrusion outside of acetabulum f. Physeal and metaphyseal irregularities g. Later, reossification and variable remodeling h. Usually loss of epiphyseal and neck height at maturity 2. Staging a. Catterall (Fig. 2.8) Fig. 2.8 Catteral classification of Perthes disease as viewed from above. 1) Central anterior involvement of head only 2) Greater central head involvement but intact medial and lateral column 3) Lateral three quarters of femoral head involved with only intact medial column; metaphyseal reaction 4) Whole-head involvement, with metaphyseal reaction and remodeling of epiphysis b. Herring Lateral Pillar Classification predicts flattening during healing (Fig. 2.9). 1) Lateral pillar is intact without radiographic change. 2) Lateral pillar is collapsed, but height is still greater than 50%. 3) Lateral pillar is collapsed to less than 50% of original height. Fig. 2.9 Herring lateral pillar classification of Perthes. a. “Head-at-risk signs” of Catterall 1) Lateral calcification 2) Lateral subluxation 3) Gage sign: Lucency proximal and distal to lateral physis 4) Metaphyseal reaction 5) Horizontal physis (meaning limb is adducted) b. Epiphyseal extrusion (Fig. 2.10) greater than 20% carries poor long-term prognosis. c. Mose sphericity: Deviation of head periphery from a perfect sphere by more than 3 mm on anteroposterior (AP) and lateral radiograph carries poor long-term prognosis. d. Stulberg rating (used after healing) assesses femoral head sphericity and its congruency with the acetabulum. There are five different stages. These have been correlated with long-term outcome (Fig. 2.11). 4. Arthrogram, magnetic resonance imaging (MRI): These are not routinely indicated but may be helpful in selected cases. 1. Hypothyroidism 2. Multiple epiphyseal dysplasia 3. Spondyloepiphyseal dysplasia Fig. 2.10 Measurement of epiphyseal extrusion. (From Green NE, Beauchamp RD, Griffin PP. Epiphyseal extrusion as a prognostic index in Legg-Calve-Perthes disease. J Bone Joint Surg Am. 1981;63(6):902 (Fig. 1). Reprinted with permission.) Fig. 2.11 Stulberg rating of outcome of healed Perthes. Stages I–II are spherical and congruent and have a low likelihood of degenerative joint disease. Stages III and IV are aspherical and congruous and have a risk of degenerative joint disease by middle age. Stage V is aspherical and incongruous, and there is a risk of degenerative joint disease before 50. Source: Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calve-Perthes disease. J Bone Joint Surg Am. 1981;63(7):1095–1108. 4. Meyer dysplasia (bilateral synchronous early childhood AVN; better prognosis) 5. Storage disorder (Gaucher disease, mucopolysaccharidoses) 6. AVN following trauma, steroids, sickle cell infarct, DDH treatment 1. There is no consensus on a protocol. However, many hips have a poor long-term natural outcome, and some hips appear to be helped by treatment. Containment is indicated if several of the following features are present: a. Head involvement greater than 50% (Catterall 3–4, Herring B or B/C border) c. Collapse or extrusion not established yet 2. Containment options a. Abduction brace or Petrie casts b. Femoral varus osteotomy c. Iliac rotational osteotomy or augmentation d. Combinations of b and c 3. Late options a. Epiphysiodesis for leg length inequality greater than 2 cm b. Valgus osteotomy for symptomatic hinge abduction c. Trochanteric transfer for persistent abductor weakness d. Epiphyseal osteotomy for femoral incongruity Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86-A (10):2103–2120 Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multi-center study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86–A (10):2121–2134 Joseph B, Nair NS, Narasimha Rao KL, Mulpuri K, Varghese G. Optimal timing for containment surgery for Perthes disease. J Pediatr Orthop. 2003;23(5):601–606 1. Transient synovitis of the hip is characterized by the acute onset of monarticular hip pain, limp, and restricted hip motion. 2. It is the most common cause of hip pain in children. 3. Child’s age is usually 1 to 4 years, but any age can be affected. 4. This condition must be distinguished from septic arthritis. 5. Gradual but complete resolution over several days to weeks is the norm. 6. Cause is unknown, but it may be immune-mediated. 1. A diagnosis of exclusion 2. Acute onset of unilateral hip pain in an otherwise healthy child 3. The patient may be afebrile or have a low-grade fever. 4. Laboratory values are nonspecific and are often within normal limits. 1. Limp and antalgic gait are common. 2. Most patients can bear weight on the involved extremity with assistance. 3. Hip is held in a flexed, externally rotated position. Restricted range of motion, especially abduction and rotation; slow movement is better. 4. Pain is not as great as with septic arthritis. 1. Laboratory tests are usually nonspecific and within normal limits, but they may help to rule out other diagnoses. 2. Peripheral white blood cell count is normal to slightly elevated. 3. Erythrocyte sedimentation rate averages 20 mm/hour but may be slightly higher. 4. Urinalysis, blood culture, rheumatoid factor, and Lyme titers results are usually within normal limits. 5. Aspiration of joint fluid is not needed if presentation is typical. If done, results are nonspecific. 1. Plain films of the hip: AP and lateral views. 2. In transient synovitis they are normal can but help rule out other diagnoses. 3. Ultrasound may be used to assess for effusion and to guide aspiration if infection cannot be ruled out clinically. 4. MRI is needed only in cases of persistent pain. 1. Septic arthritis 2. Osteomyelitis in the femoral neck or pelvis 3. Tuberculous arthritis 4. Psoas abscess 5. Other muscle infection about the hip 6. Juvenile rheumatoid arthritis 7. Idiopathic chondrolysis 8. Acute rheumatic fever 9. Legg-Calvé-Perthes disease 10. Tumor 11. Sacroiliac joint infection 1. Bed rest at home if diagnosis is clear or in hospital if further workup is needed. 2. Nonsteroidal anti-inflammatory drugs (NSAIDs) 3. Prompt improvement should be seen. 4. Activity as tolerated when clinically improved 1. Good; no clear evidence of increased risk of AVN Dobbs Matthew B. Transient synovitis of the hip. In Morrissy RT, Weinstein SL, eds. Lovell and Winter’s pediatric orthopaedics. Vol 2. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1142–1147. Haueisen DC, Weiner DS, Weiner SD. The characterization of “transient synovitis of the hip” in children. J Pediatr Orthop. 1986;6(1):11–17 Johnson K, Haigh SF, Ehtisham S, Ryder C, Gardner-Medwin J. Childhood idiopathic chondrolysis of the hip: MRI features. Pediatr Radiol. 2003;33(3):194–199 Kocher MS, Mandiga R, Murphy JM, et al. A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg Am. 2003;85-A (6):994–999 Kocher MS, Mandiga R, Zurakowski D, Barnewolt C, Kasser JR. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A (8):1629–1635 Landin LA, Danielsson LG, Wattsgård C. Transient synovitis of the hip. Its incidence, epidemiology and relation to Perthes’ disease. J Bone Joint Surg Br. 1987;69(2):238–242 1. Incidence: 2 to 10 per 100,000 a. Higher in males than females b. Higher in African Americans c. 20% have bilateral involvement at presentation d. 20% more bilateral later 2. Etiologic factors a. Obesity b. Trauma: Mild or severe c. Endocrine disorders: Hypothyroidism, hypogonadism, rickets, renal failure e. Family history f. Radiation 1. Loder classification • Stable: Able to bear weight (even with crutches) • Unstable: Unable to bear weight 2. Chronologic • Acute: Symptoms of less than 3 weeks’ duration • Chronic: Symptoms for 3 weeks or longer 3. Severity • Grade I: Less than 33% slip of epiphysis on metaphysis • Grade II: 33 to 50% slip • Grade III: More than 50% slip • “Pre-slip”: Symptoms are present in patient at risk, but no observable slip is seen; MRI may be positive. 1. Age 9 to 14 years, most common 2. Antalgic limp 3. Pain in the thigh, knee, or hip 4. Leg externally rotated during gait and at rest 5. Internal rotation less in flexion than extension 6. Seasonal variation: Highest rates in September, lowest in March because of sunlight and vitamin D cycles 7. Age–weight test: If age is younger than 10 or older than 16 years or weight is less than 50 percentile, suspect nonidiopathic slip and perform an endocrine workup. 8. Height test: If height is below 10th percentile for age, risk of atypical slip is increased increased; perform an endocrine workup. 1. Slip is best seen on lateral view. 2. AP view a. Physeal widening, irregularity b. Decreased epiphyseal height d. Chondrolysis–joint space narrowing may be seen before treatment (rare). 1. Immediate weight relief (bed rest) 2. Traction for acute slip for comfort or reduction if severe (optional). 3. Fixation in situ a. Single screw is centrally placed within physis. b. Second screw may be used if first is not perfect or if slip is severe. 4. Realignment: Main indication is the patient who is dissatisfied with limb deformity resulting from slip; it is not commonly needed. a. Open realignment and pinning of acute slip b. Cuneiform osteotomy just below physis c. Base of neck osteotomy: some series show high AVN rate. d. Subtrochanteric osteotomy (Southwick) 5. Prophylactic contralateral pinning a. Cost–benefit studies show prophylactic pinning is justifiable (at the surgeon’s discretion). b. Main indication is a patient in whom diagnosis of late contralateral SCFE may be missed as a result of impaired communication or follow-up. c. Also valid option in patients with SCFE before growth spurt (in girls younger than 10 years, in boys younger than 12 years) d. If triradiate cartilage is closed or in a girl older than 13 years or in a boy older than 14 years, there is a low risk of subsequent slip (~7%). 1. Chondrolysis: Affects 5% or less and usually improves with time and physical therapy 2. AVN a. Greater in unstable or acute slips b. May be focal or complete c. Some healing is possible in young patients. d. Some patients can function 10 to 20 years with AVN before salvage is needed. Carney BT, Weinstein SL, Noble J. Long-term follow-up of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1991;73(5):667–674 Kocher MS, Bishop JA, Hresko MT, Millis MB, Kim YJ, Kasser JR. Prophylactic pinning of the contralateral hip after unilateral slipped capital femoral epiphysis. J Bone Joint Surg Am. 2004;86-A (12):2658–2665 Koenig KM, Thomson JD, Anderson KL, Carney BT. Does skeletal maturity predict sequential contralateral involvement after fixation of slipped capital femoral epiphysis? J Pediatr Orthop. 2007;27(7):796–800 Loder RT, Aronsson DD, Weinstein SL, Breur GJ, Ganz R, Leunig M. Slipped capital femoral epiphysis. Instr Course Lect. 2008;57:473–498 Loder RT, Starnes T, Dikos G. Atypical and typical (idiopathic) slipped capital femoral epiphysis: reconfirmation of the age-weight test and description of the height and age-height tests. J Bone Joint Surg Am. 2006;88(7):1574–1581 Riad J, Bajelidze G, Gabos PG. Bilateral slipped capital femoral epiphysis: predictive factors for contralateral slip. J Pediatr Orthop. 2007;27(4):411–414 Schultz WR, Weinstein JN, Weinstein SL, Smith BG. Prophylactic pinning of the contralateral hip in slipped capital femoral epiphysis: evaluation of long-term outcome for the contralateral hip with use of decision analysis. J Bone Joint Surg Am. 2002;84-A (8):1305–1314 Yildirim Y, Bautista S, Davidson RS. Chondrolysis, osteonecrosis, and slip severity in patients with subsequent contralateral slipped capital femoral epiphysis. J Bone Joint Surg Am. 2008;90(3):485–492 A varus deformity of femoral neck that is usually progressive. Incidence is 1 in 25,000. Thirty percent of cases are bilateral. 1. Femoral neck shortened and in varus 2. Inverted “Y” appearance of physis as a result of shear plane through metaphysis 3. Triangular fragment on inferior femoral neck 4. Decreased femoral anteversion 5. Mild acetabular dysplasia 1. Abnormal gait caused by abductor weakness and leg-length inequality 2. Activity-related hip pain 3. Length inequality if unilateral: Usually less than 2.5 cm 1. Cleidocranial dysplasia 2. Metaphyseal dysplasia 3. Morquio syndrome Fig. 2.12 Hilgenreiner–epiphyseal angle; normal is less than 25 degrees. Note “inverted Y” appearance of physis on involved right hip. 1. Observe if Hilgenreiner-epiphyseal angle is less than 45 degrees (Fig. 2.12) 2. Valgus-derotation osteotomy if greater than 45 degrees and progressive and symptomatic or if greater than 60 degrees at diagnosis Weinstein JN, Kuo KN, Millar EA. Congenital coxa vara: a retrospective review. J Pediatr Orthop. 1984;4(1):70–77 A spectrum of congenital femoral anomalies including a temporary or permanent discontinuity in the proximal femur 1. A: Femur is in continuity but appears discontinuous early. 2. B: Varus/shortening is more extreme, but most ossify later. Fig. 2.13 Proximal focal femoral deficiency: Aitkin classification. 3. C: Small acetabulum but no femoral head 4. D: No femoral head or acetabulum Other classification systems exist but are less widely used. 1. 15% are bilateral (most type D). 2. More than 50% have other lower extremity anomalies, most commonly fibular hemimelia. 3. Affected limb is a constant proportion to the length of the normal limb. 1. Limb-length inequality (if unilateral) 2. Pelvic–femoral instability 3. Malrotation of lower extremity (flexion–abduction–external rotation) 4. Proximal muscle weakness 1. Bilateral a. Patients walk without prostheses if feet are all right. b. Nonfunctional feet may need surgery. c. Extension prostheses may be used when desired to increase height. 2. Unilateral a. Hip abnormalities: Valgus osteotomy and lengthening for types A and B; consider femoropelvic arthrodesis for type D versus containment of thigh segment in a prosthesis. b. Knee: Offer rotationplasty if foot and ankle are strong and positioned distal to the contralateral knee. Syme disarticulation is another option. Both are combined with fusion of anatomic knee to increase lever arm. c. Foot: Prosthesis equalizes length and provides plantigrade foot (Fig. 2.13). Aitken GT. Proximal Femoral Focal Deficiency: Definition, Classification and Management. National Academy of Sciences Symposium, Washington, D.C., 1969 Fowler EG, Hester DM, Oppenheim WL, Setoguchi Y, Zernicke RF. Contrasts in gait mechanics of individuals with proximal femoral focal deficiency: Syme amputation versus Van Nes rotational osteotomy. J Pediatr Orthop. 1999;19(6):720–731 Kalamchi A, Cowell HR, Kim KI. Congenital deficiency of the femur. J Pediatr Orthop.1985;5(2):129–134 PubMed A spectrum of anomalies that may involve the bladder, pelvis, intestinal tract, and external genitalia. 1. The most common form is “classic” exstrophy, which involves a widened pelvis with an anterior diastasis, an open bladder, and a complete epispadias. 2. The mildest form is epispadias, which may have a closed bladder but widened pelvic symphysis. 3. The most pronounced expression of this spectrum is cloacal exstrophy, which usually involves all of the above as well as omphalocele and a lumbosacral neural tube defect. It often includes anomalies of the spine and extremities. 4. The incidence of bladder exstrophy is around 1:25,000 live births. Males are more commonly affected. 1. Defect in lower abdominal wall 2. Open bladder and urethra 3. In cloacal exstrophy, abdominal wall defect is larger and lower intestinal tract is exposed. 4. A spinal and a neurological examination should be performed. Often in cloacal patients there is lipomeningocele or myelomeningocele. Hip dislocation, foot deformity, or partial sacral agenesis may occur. Separation of pubic bones, typically about 4 to 5 cm at birth, and increases steadily with age (Fig. 2.14). The iliac wings are externally rotated and “flattened.” The ischiopubic bones are slightly underdeveloped. The hips themselves rarely show dysplasia. 1. The urologist usually performs the reconstruction in several stages, including closure of the bladder and lower abdominal wall soon after birth, followed by epispadias closure at the same time or at a later date. Surgery to achieve continence is commonly performed after the age at which children are normally continent and may consist of bladder neck suspension. 2. Orthopedic surgery of the pelvic deformity is indicated only if it is needed to achieve urologic goals. Fig. 2.14 Schematic representation of pelvic differences in classic exstrophy versus normals in the transverse plane. 3. In the neonatal period, pubis may be approximated manually and temporarily held with suturing. 4. In an older child, iliac osteotomy is indicated either anteriorly or posteriorly. The hip function in the untreated patient with classic bladder exstrophy is good. Children walk at a normal age, although they have an increased external foot-progression angle. This becomes less pronounced over time. Adults with exstrophy have an increased incidence of pain in the region of the sacroiliac joints. One natural history study suggests an increased incidence of degenerative disease of the hip in patients with uncorrected exstrophy. Patients with exstrophy are usually fertile. Aadalen RJ, O’Phelan EH, Chisholm TC, McParland FA Jr, Sweetser TH Jr. Exstrophy of the bladder: long-term results of bilateral posterior iliac osteotomies and two-stage anatomic repair. Clin Orthop Relat Res. 1980;151(151):193–200 Jani MM, Sponseller PD, Gearhart JP, Barrance PJ, Genda E, Chao EY. The hip in adults with classic bladder exstrophy: a biomechanical analysis. J Pediatr Orthop. 2000; 20(3):296–301 Okubadejo GO, Sponseller PD, Gearhart JP. Complications in orthopedic management of exstrophy. J Pediatr Orthop. 2003;23(4):522–528 Sponseller PD, Bisson LJ, Gearhart JP, Jeffs RD, Magid D, Fishman E. The anatomy of the pelvis in the exstrophy complex. J Bone Joint Surg Am. 1995;77(2):177–189 Sponseller PD, Jani MM, Jeffs RD, Gearhart JP. Anterior innominate osteotomy in repair of bladder exstrophy. J Bone Joint Surg Am. 2001;83-A (2):184–193 A focal varus deformity of the proximal tibia resulting from overload causing disordered medial growth. Tibia vara may have onset in the infantile, juvenile, or adolescent period (Table 2.1). 1. Normal alignment of the lower extremity is shown in Chapter 1’s Fig. 1.38. 2. In infantile tibia vara, changes involve physeal depression with lucency and beaking of the corresponding metaphysis and epiphysis. These changes have been staged 1 through 7 by Langenskjold (Fig. 2.15). Fig. 2.15 Langenskjold stages of infantile tibia vara. (From Langenskiold A. Tibia vara. (Osteochondrosis deformans tibiae.) A survey of 23 cases. Acta Chir Scand. 1952;103(1):1–22 (Fig. 5). Reprinted with permission.) 3. Infants under age 2 years have inadequate ossification to assign Langenskjold stages. As an alternate means of early diagnosis, the metaphyseal–diaphyseal angle may be drawn (Fig. 2.16). An angle of greater than 16 degrees is diagnostic of infantile Blount disease. An angle of less than 11 degrees is rarely seen in patients with Blount disease and rules this diagnosis out. 4. Medial physeal slope greater than 60 degrees may predict recurrent bowing after osteotomy (Fig. 2.17). 5. Medial plateau depression greater than 30 degrees may be seen in neglected infantile tibia vara (Fig. 2.18). Fig. 2.16 Metaphyseal-diaphyseal angle used to detect tibia vara in very young children. High diagnostic likelihood if angle is greater than 11 to 16 degrees. Fig. 2.17 Metaphyseal slope is used to predict the risk of recurrent deformity. Values over 60 degrees are at increased risk. Fig. 2.18 Medial plateau depression is unique to infantile tibia vara. If greater than 25 degrees, the medial side may need to be selectively elevated. 6. Mechanical axis deviation quantifies the degree of varus. The axis line is drawn from the center of the hip to the center of the ankle. The degree of varus (or valgus) is quantified as follows (Fig. 2.19). 1. Rickets: Many types 2. Achondroplasia 3. Metaphyseal chondrodysplasia (Schmidt) 4. Trauma or infection of medial physis 5. Physiologic bowing 6. Focal fibrocartilaginous dysplasia 1. Infantile a. Observe or brace (day versus night) b.
 Lower-Limb Length Inequality
Lower-Limb Length Inequality
Principles
Congenital Causes
Acquired Causes
Measurement
Treatment
Bibliography
 Developmental Dysplasia of the Hip
Developmental Dysplasia of the Hip
Principles
Risk Factors in History
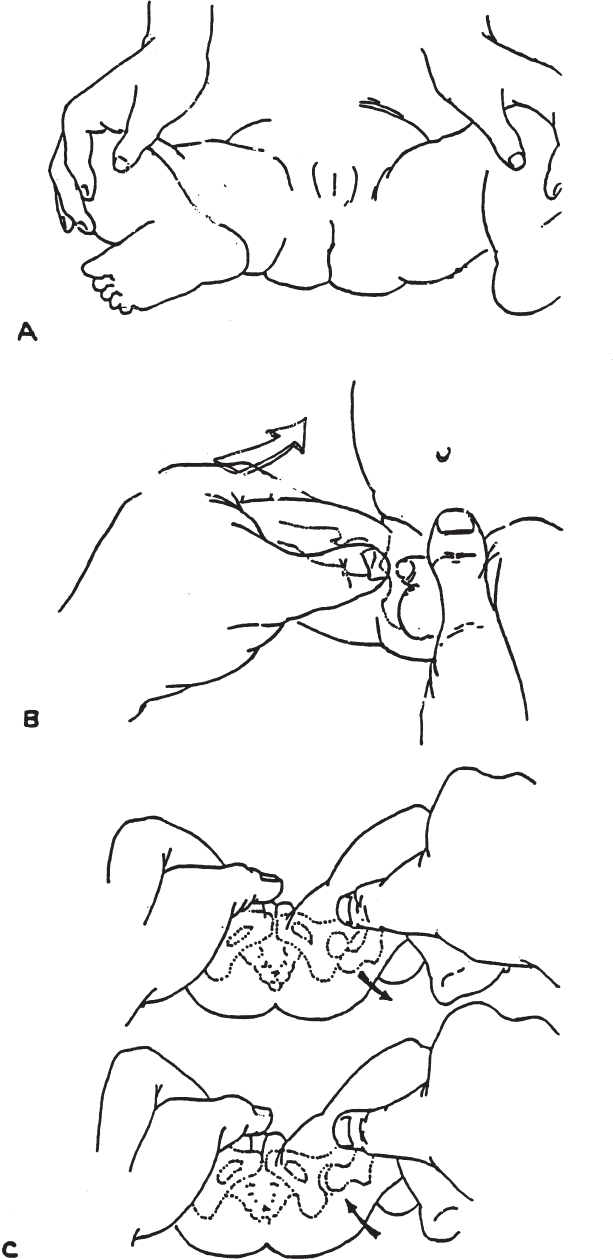
Physical Examination for Developmental Hip Dysplasia in the Newborn
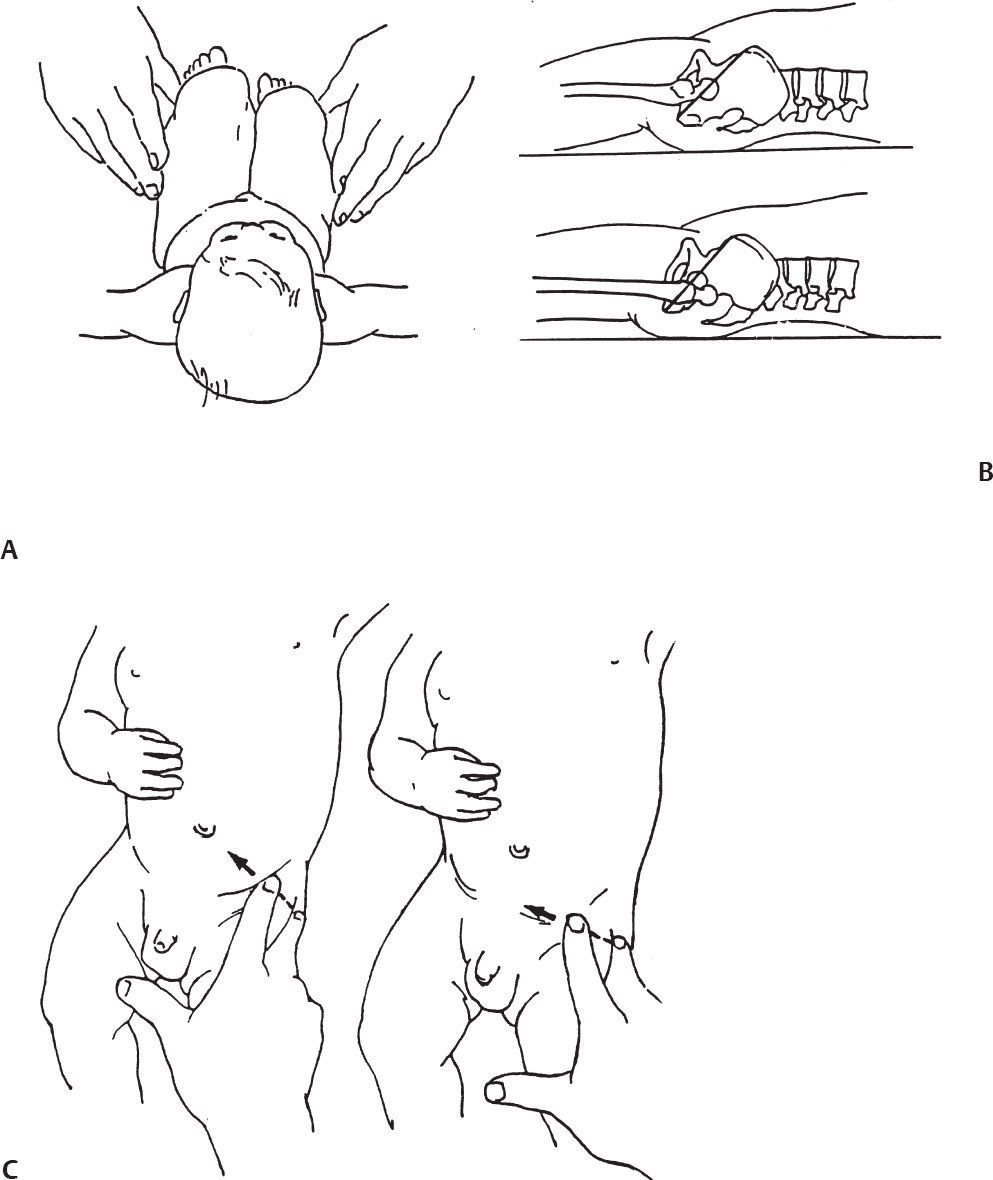
Evaluation of the Older Child for Developmental Dysplasia of the Hip
Ultrasound for Hip Dysplasia
Principles
Coronal View
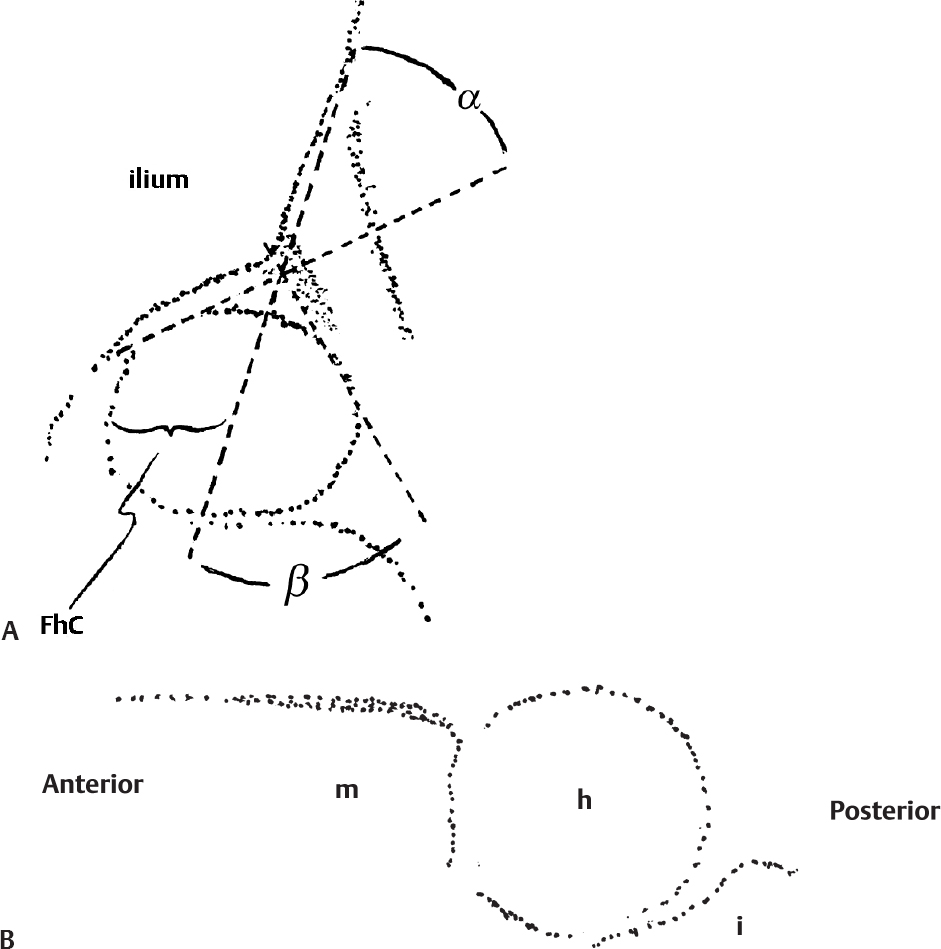
Transverse View
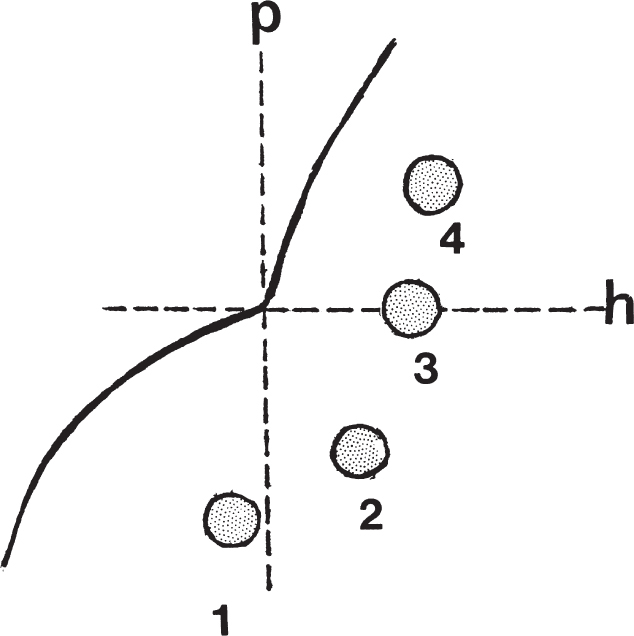
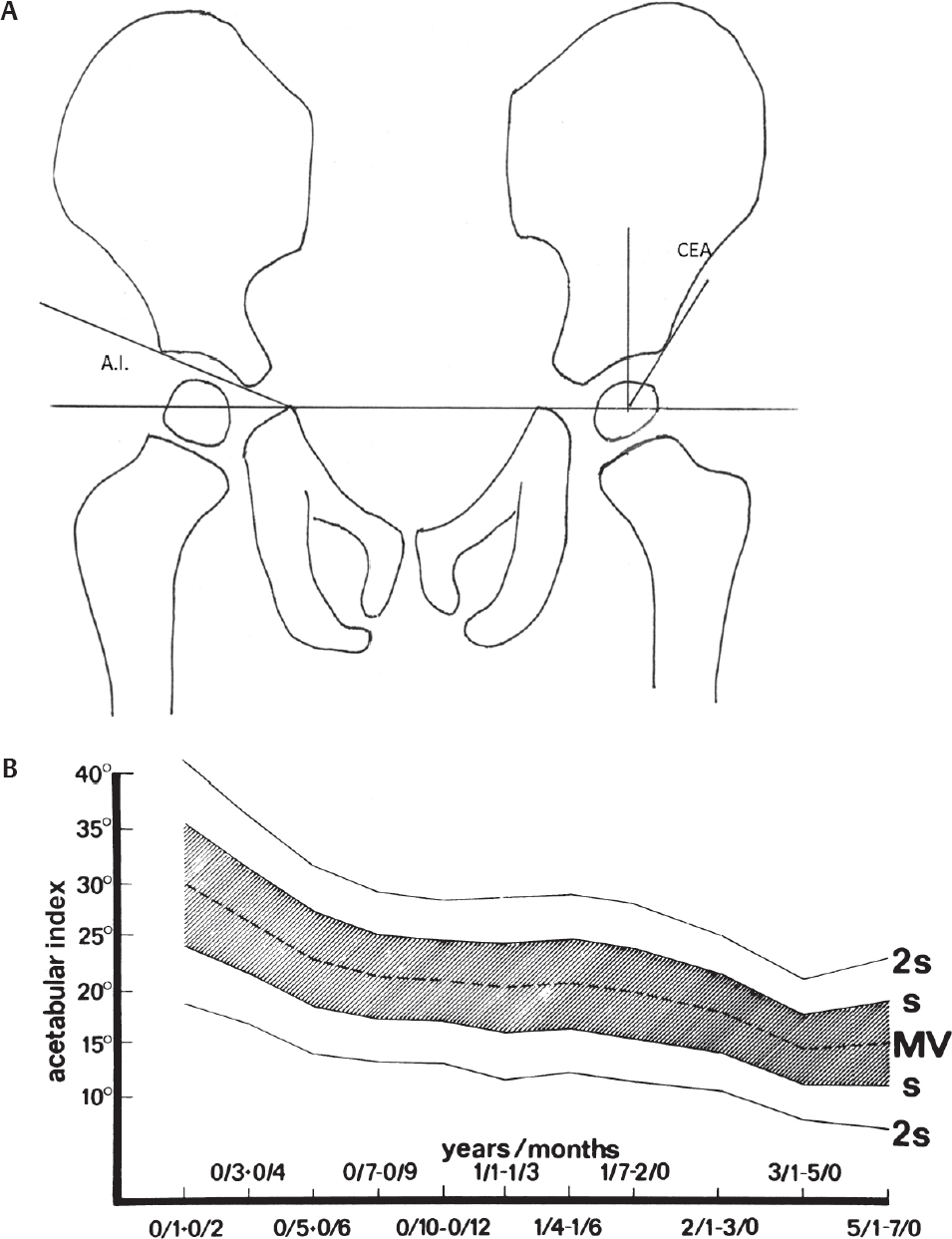
Radiographic Evaluation of Hip Dysplasia
Bibliography
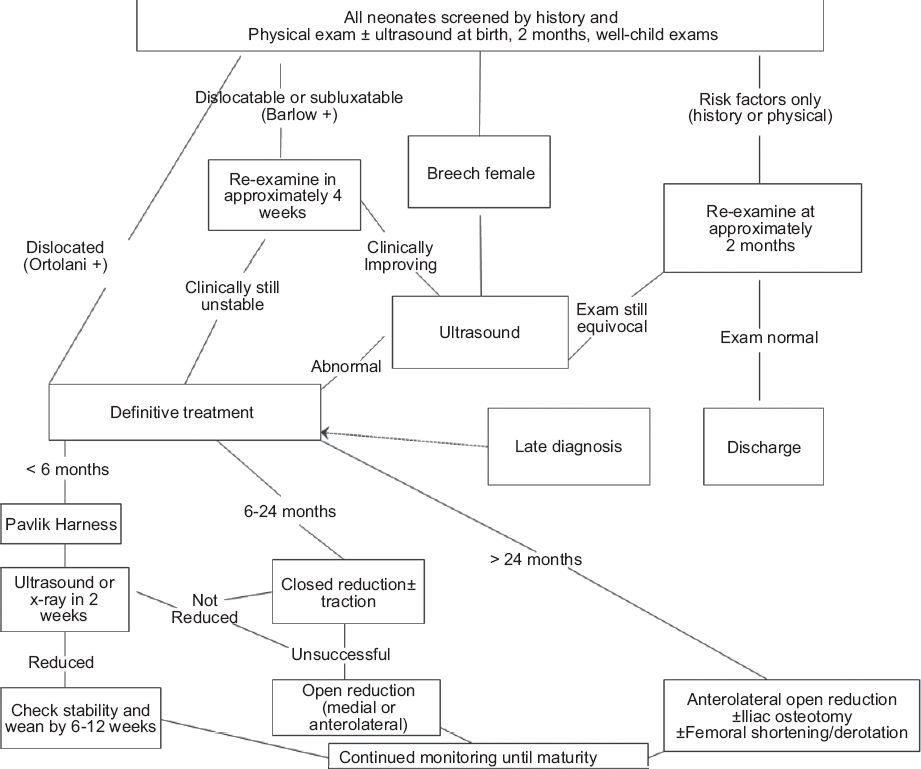
Management of Hip Dysplasia
Bibliography
 Legg–Calve–Perthes Disease
Legg–Calve–Perthes Disease
Symptoms
Signs
Radiographic Findings
Differential Diagnosis
Treatment
Bibliography
 Transient Synovitis of the Hip
Transient Synovitis of the Hip
Overview
Diagnosis
Physical Examination
Laboratory Tests
Imaging
Differential Diagnosis
Treatment
Prognosis
Bibliography
 Slipped Capital Femoral Epiphysis
Slipped Capital Femoral Epiphysis
Background
Classification
Clinical Presentation
Radiographic Features
Treatment
Complications
Bibliography
 Developmental Coxa Vara
Developmental Coxa Vara
Radiographic Features
Signs and Symptoms
Differential Diagnosis
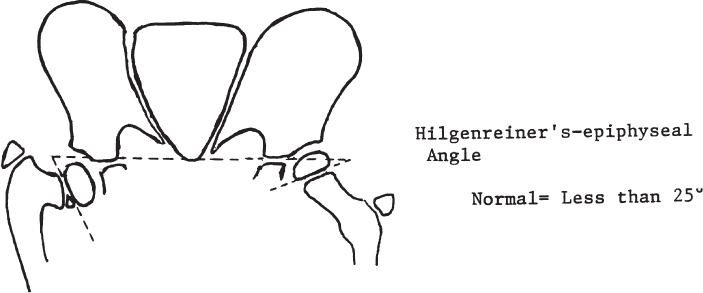
Treatment
Bibliography
 Proximal Focal Femoral Deficiency
Proximal Focal Femoral Deficiency
Classification (Aitken) (Fig. 2.13)
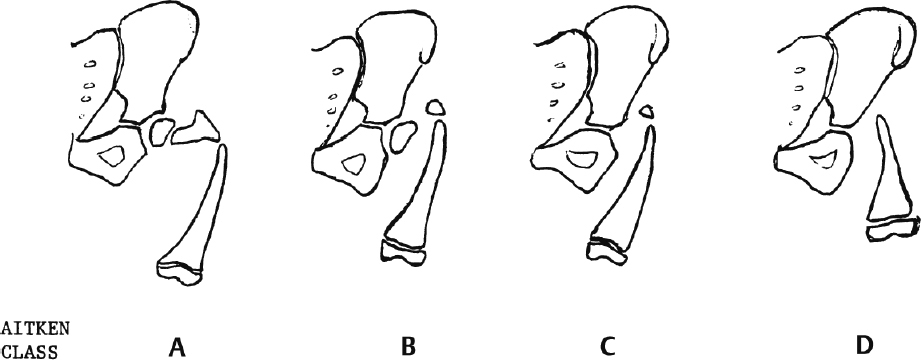
Characteristics
Problems
Treatment
Bibliography
 Bladder Exstrophy
Bladder Exstrophy
Clinical Features
Radiographic Features
Treatment
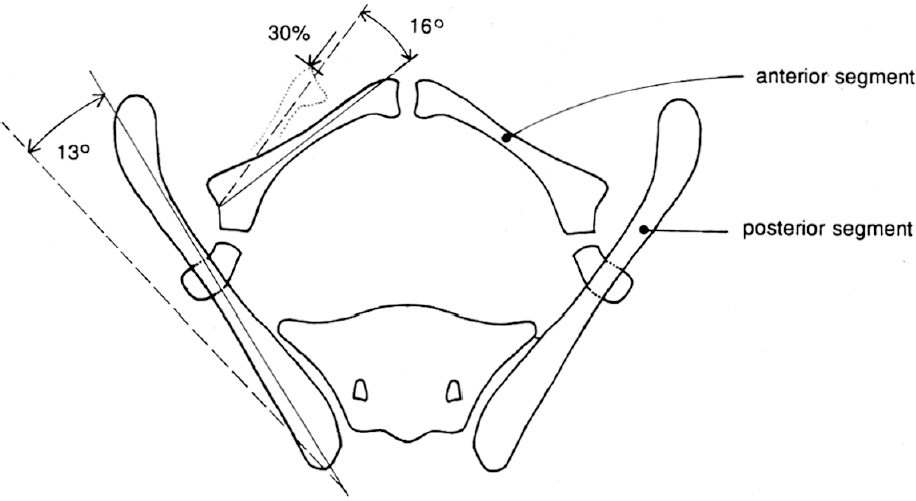
Prognosis
Bibliography
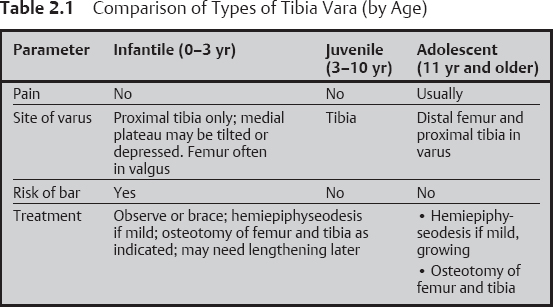
 Tibia Vara
Tibia Vara
Radiographic Features
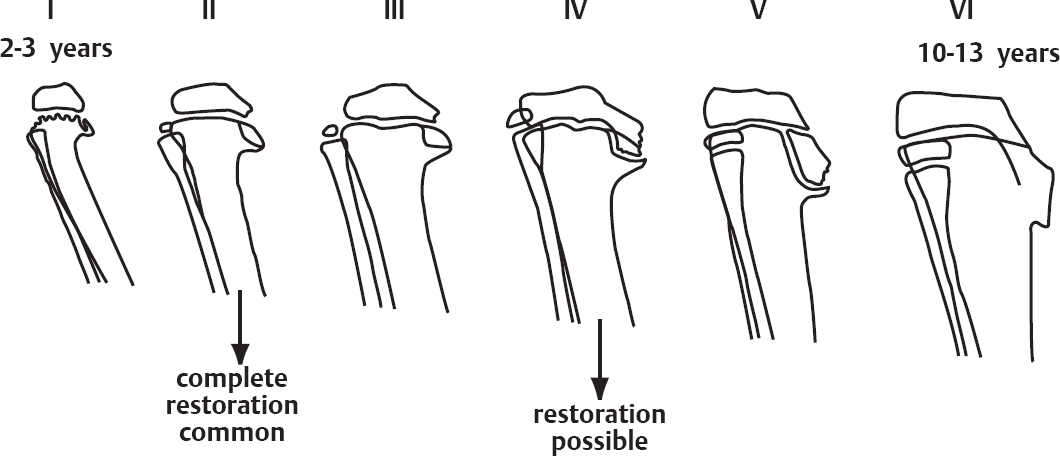
Differential Diagnosis
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree