Fig. 1
AP and lateral view of the right knee. Significant osteolyses was shown on both the femur and tibia (Arrows)
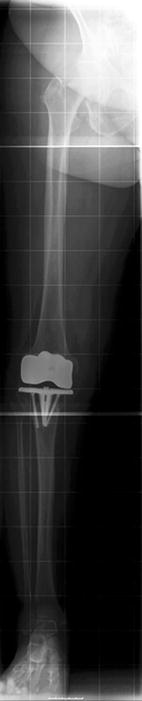
Fig. 2
The long leg-X-ray showed significant varus malalignment due to component subsidance predominantly at the medial tibial plateau
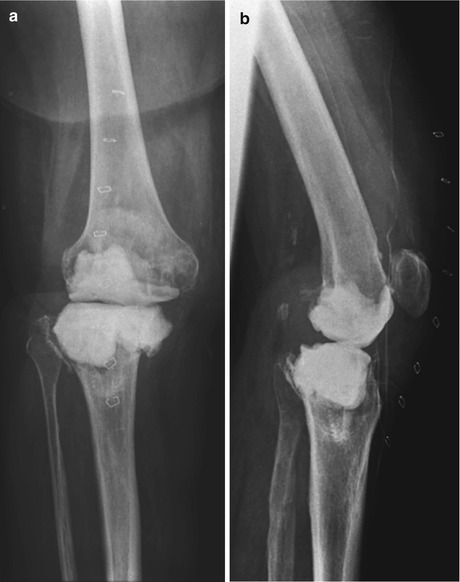
Fig. 3
AP (a) and lateral (b) view after removal of the TKR components. Two spacers were inserted, one at the femoral and the other at the tibia site
Discussion
What are the surgeon’s questions?
1.
Single-stage or two-stage revision?
2.
How long should the Palacos® spacer stay in place?
3.
How long antibiotics should be given?
4.
How should be the aseptic condition proved?
5.
What type of implant should be used?
6.
What type of bony augmentation is required?
1.
Two-stage revision was considered in this case, as it was an acute onset infection and bacteriological testing was not possible before surgery. Severe osteolysis, especially on the tibial site, is a sign for a longer history. The osteolysis might be caused due to infection; however, primary aseptic loosening may also be considered and infection developed secondarily. Aseptic loosening and severe cyst formation at the proximal tibia component have been reported after cementless tibial fixation [1]. The review of the literature has shown that there is still a controversial discussion about single-stage and two-stage revision surgery. Many studies did not find significant difference, but the level of evidence of these studies is rather low [2, 3]. However, more recent analysis of the literature has shown that single-stage revision surgery might be better in terms of functional outcome without a difference in reinfection rate [4].
2.
The spacer was left in place for 6 weeks. IV antibiotics were given for seven days and then changed to oral (rifampicin and cefuroxime). The treatment with antibiotics was finished after three weeks. After an interval of 2 weeks, biopsies were taken arthroscopically. Five biopsies were taken from all compartments of the knee. After approval that the biopsies were sterile, the patient was scheduled for reimplantation of a new prosthesis.
The spacer should not be in place longer than 6–8 weeks because the Palacos® cement causes severe arthrofibrosis, which may cause poor functional outcome. For that reason and the fact that the antibiotic level in the surrounding tissue drops significantly after 10 days, one should rather keep the space in place for less than 2 weeks. If necessary, the spacer should be changed after 2 weeks. No difference has been reported in the outcome between static and articulating spacer [5].
3.
Antibiotics should be given in revision surgery for a course of 6 weeks. However, the duration of antibiotics in revision surgery depends from the type of microorganism.
4.




It has been shown that joint fluid aspiration has a sensitivity of 72.5 % and specificity 92.2 % [6]. The biopsy technique revealed a sensitivity of 100 % and a specificity of 98.1 %. We also monitor the WBC and CRP. The serum CRP has a sensitivity of 91 %, specificity of 86 %, a positive predictive value of 74 %, and a negative predictive value of 95 % [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








