Chapter 34 Diabetes Mellitus
Definition and Pathogenesis
Although elevated fasting glucose remains the earliest and most readily detectable sign of the onset of diabetes mellitus (DM), the metabolic errors of diabetes are likely disordering the utilization of all substrates long before hyperglycemia becomes apparent. Both glucose and fatty acids are energy substrates oxidized by muscles, including the myocardium, for adenosine triphosphate (ATP) generation. The relative lack of insulin in DM leads to lipolysis or the release of fatty acids stored in adipose tissue, resulting in increased free fatty acid (FFA) levels. FFAs then compete with glucose for oxidative disposal or storage in muscles. This substrate competition further promotes hyperglycemia. During conditions of marked insulin deficiency, a massive efflux of FFAs from adipose tissue can precipitate diabetic ketoacidosis because of the incompletely oxidized FFA degradation products β-hydroxybutyrate and acetoacidic acid. Defects in FFA removal from lipoproteins account for the dyslipidemias implicated in the acceleration of atherogenesis in the diabetic state. When fatty acids packaged into triglycerides are not properly degraded in tissues, their intracellular accumulation causes lipotoxicity, which in turn impairs cellular function, including the beta cell of the pancreatic islets.
Classification and Diagnosis
The diagnosis of GDM is made during pregnancy using specific criteria discussed later.
Epidemiology
Types
Patients with type 1 diabetes make up only 5% to 10% of the diabetic population; the remaining 90% to 95% have type 2. Usually, type 1 diabetic patients first manifest their illness in childhood or adolescence, with about 1 in 4000 children having DM; this includes a few young people with the increasingly prevalent maturity-onset diabetes of the young (MODY), although this form may not manifest until adulthood. MODY is classified under “other specific types,” which include a heterogeneous group of conditions (specific genetic conditions, surgery, drugs, malnutrition, infections) that result in diabetic manifestations and symptomatology (Table 34-1). These other types are found in 1% to 5% of the diabetic population. GDM is by definition temporal and is categorized separately; 3% to 6% of all pregnant women develop GDM, and 40% to 60% of women with GDM develop diabetes, usually type 2, within a decade of the pregnancy.
Table 34-1 Other Specific Types of Diabetes
| Cause or Category | Examples |
|---|---|
| Genetic defects of beta-cell function | Maturity-onset diabetes of the young (MODY) |
| Genetic defects in insulin action | Lipoatrophic diabetes |
| Diseases of exocrine pancreas | Pancreatitis, cystic fibrosis |
| Endocrinopathies | Acromegaly, Cushing’s syndrome, hyperthyroidism |
| Drug or chemical induced | Nicotinic acid, thiazides |
| Infections | Congenital rubella, cytomegalovirus |
| Uncommon forms of immune-mediated diabetes | Antibody against insulin receptor |
| Other genetic syndromes occasionally associated with diabetes | Down, Klinefelter’s, Turner’s |
From Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(suppl 1):4-20.
Pathophysiology
Common Mechanisms
The clinical characteristics of type 1 and 2 diabetes are shown in Table 34-2. However, diabetic classification and nomenclature have required frequent revisions over the years as the pathogenesis of DM is better understood and changes in culture and human behavior continue to alter its clinical expression. The finding of hyperglycemia on routine examinations of apparently healthy individuals has resulted in a new DM subclass that does not lend itself to easy classification. The finding of anti–islet cell antibodies designates this group as latent autoimmune diabetes of adults (LADA). This group mostly consists of young adults who have a prolonged subclinical development of DM. The diabetes may remain indolent or more rapidly deteriorate into classic insulin-dependent type 1 diabetes, or it may continue to evolve into insulin resistance and dependency coincident with weight gain. In this case, LADA patients are clinically indistinguishable from type 2 patients but have continuing risk of deteriorating to insulin dependency. Thus a patient recognized to have DM may have diverse etiopathogenic mechanisms causing insulin dependency and resistance resulting in hyperglycemia.
Table 34-2 Characteristics of Types 1 and 2 Diabetes Mellitus
| Sign | Type 1 | Type 2 |
|---|---|---|
| Age of onset | Usually childhood and adolescence | About 40 years, increasing with age; adolescence with childhood obesity |
| Family history, concordance in twins | Uncommon; 50% before age 40 | Common, 95% afterage 40 |
| Diagnostic markers | Anti-GAD, undetectable C peptide | C peptide variable: high with insulin resistance; low values improve with glycemic control. |
| Other associated disorders | Primary hypothyroidism, celiac disease, other autoimmune endocrine disorders | Metabolic syndrome |
| Glycemic control, ketosis | Marked brittleness, ketosis prone with hyperglycemia | Stable hyperglycemia without ketosis |
| Treatment requirement | Absolute need for insulin; coincident genetic insulin resistance that might respond to oral drugs | Therapeutic lifestyle changes with and without oral agents; insulin is required when other measures fail. |
GAD, Glutamic acid decarboxylase.
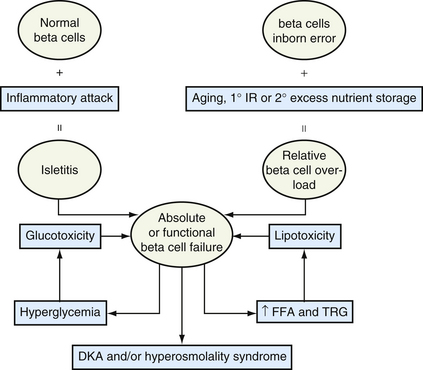
Adolescents with significant obesity who develop type 2 diabetes are younger at presentation than usual, so age of onset of type 2 now overlaps with type 1 diabetes. Because their obesity is often intractable, their marked state of insulin resistance at DM onset eventually becomes a state of insulin deficiency. When DM patients develop marked hyperglycemia and dyslipidemia, the resulting glucotoxicity and lipotoxicity cause systemic cellular dehydration along with triglyceride accumulation in skeletal muscle. These acquired defects further accentuate defects in beta-cell insulin secretion and muscle insulin sensitivity. Pathogenic mechanisms may differ (Fig. 34-1), but the resulting glucotoxicity and lipotoxicity so impair insulin secretion and effectiveness that diabetes from any mechanism may lead to similar metabolic crises: diabetic ketoacidosis with or without hyperosmolality.
Mechanisms Underlying Other Specific Types of Diabetes
A diverse array of genetic defects or acquired clinical disorders can impair beta-cell function or insulin sensitivity, resulting in hyperglycemia and the diagnosis of diabetes mellitus (see Table 34-1). These patients generally appear to have type 2 diabetes at presentation and manifest varying degrees of diabetic severity during the course of their primary disorder.
Presentation
Type 2 Diabetes
The typical indolent course of type 2 diabetes can be further extended by a therapeutic lifestyle. However, this does not mean it is any less serious than type 1 as a state of accelerated vascular aging. Unfortunately, treating middle-aged and older patients with long-term type 2 diabetes with aggressive pharmacologic methods to correct hyperglycemia has failed to improve cardiovascular outcomes (ACCORD, 2008; VADT, 2009). Improved glycemic control in type 2 diabetes, as in type 1, has been shown to preclude progression of small-vessel disease and nerve function disorder to diabetic retinopathy, nephropathy, and neuropathy (UKPDS, 1998a).
Management
Behavioral Therapy
KEY TREATMENT
Glucose Monitoring
KEY TREATMENT
Nutrition and Diet
Exercise
In addition to dietary guidelines, the U.S. Department of Agriculture (USDA, 2005) also recommends daily exercise activities for weight loss and health maintenance. The difference in these objectives is related to the duration and the intensity of exercise. The advocacy of exercise, as with dieting and food choices, has become an American industry. Exercise is an essential intervention in the diabetic lifestyle. Studies show that exercise activities even without weight loss result in consistently beneficial and safe outcomes. In type 2 diabetic patients with HbA1c of less than 9%, exercise can lower this indicator by 1%. A similar effect in type 1 diabetes has not been demonstrated, despite improved insulin sensitivity. However, both type 1 and 2 patients show improved serum lipid profile and increased fibrinolytic proteins after exercise. Based on its effects in improving athletic performance, exercise in a diabetic patient encourages good cardiac function with increased circulation to muscles and the periphery.
Exercise Prescription
For patients under age 40, the maximum heart rate (HR) is calculated simply by subtracting the patient’s age from 220; for a 35-year-old patient, maximum HR would be 220 − 35, or 185 beats/min. Within a 30-minute exercise regimen, after a 5-minute warm-up, there should be 20 minutes of exercise in the pulse range of 111 to 124 beats/min (60%-67% of 185), followed by another 5 minutes of a cool-down phase. With more conditioning, the patient should aim to exercise in the range of 75% to 85% (139-157 beats/min). For patients over 40, the formula for maximum HR is 208 − (0.7 × age); for a 45-year-old patient, 208 – (0.7 × 45) = 208 − 32 = 176 beats/min, with the calculated HR guideposts of 106 (60%), 118 (67%), 132 (75%), and 150 (85%) beats/min.
Pharmacologic Therapies
Glycemic Targets
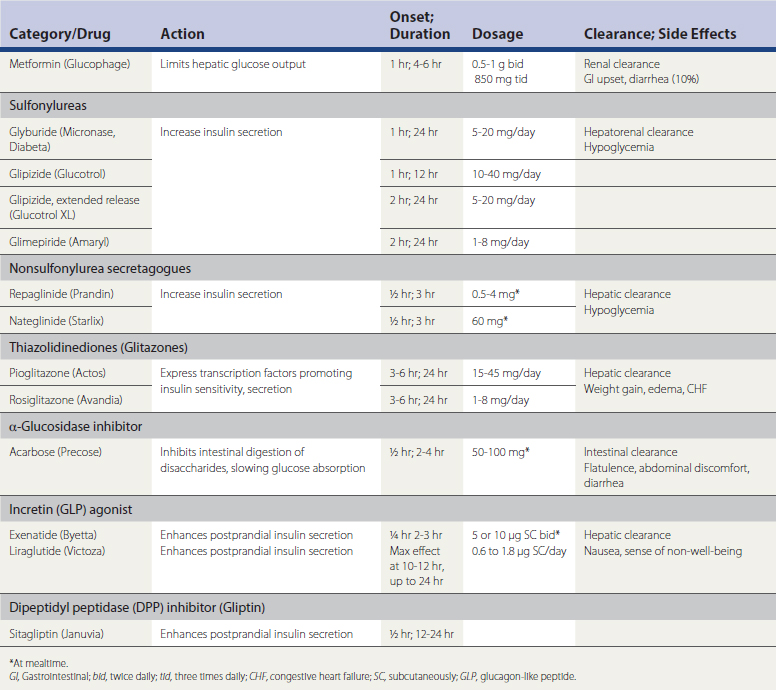
Table 34-3 lists the classes of antidiabetic drugs, mode and duration of action, effectiveness, and side effects. The introduction of the newer agents now allows the clinician to consider drugs that address a specific etiopathogenic mechanism. If the patient is thought to have a type 1 presentation, insulin at the onset is the logical and safest treatment, as discussed later. Oral medications, at least in the short term, should be advised for type 2 diabetic presentations. Behavioral therapies can produce dramatic improvement even at higher HbA1c values, but a FSG over 200 mg/dL indicates marked insulin insufficiency, and some form of drug therapy (including insulin) is necessary to reduce such values expeditiously. Instituting drug therapy should not deemphasize behavioral therapies; as noted, once the inertia of gluco/lipotoxicity is overcome with drugs, effective behavioral therapies could control DM indefinitely.
Oral Agents
Sulfonylureas and Meglitinides
The sulfonylureas are the oldest class of drugs and were found to be as safe and effective as insulin in the UKPDS. They shut down membrane potassium ion (K+) channels, allowing membrane calcium (Ca++) channels to open, thereby establishing a calcium gradient that stimulates insulin secretion. In the initial phase of treatment, insulin levels are higher than the previous state following the drug’s stimulatory effects. As insulin resistance factors are improved by controlling hyperglycemia, insulin levels fall into a normal range. Treatment is usually effective for 5 to 10 years, depending on the efficacy of behavior treatment and the underlying diabetic progression. The therapeutic duration of sulfonylureas can be prolonged if an overnight insulin preparation is added to control FSG level.
Alpha-Glucosidase Inhibitors
The α-glucosidase inhibitors have been the least utilized of the oral medications available in the United States because of their side effects, not because of their lack of efficacy. The agents interfere with intestinal digestion of complex carbohydrates, slowing the absorption of glucose and limiting the beta-cell insulin response, thus lowering insulin levels. Their disadvantage is that they require administration with meals and often cause postprandial abdominal discomfort and flatulence. Patients who can tolerate these agents can experience HbA1c reductions comparable to other oral agents. However, most patients eventually request a change in medication. These drugs may be better tolerated at the onset of diabetes and prediabetic phase of diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree