Developmental Hip Dysplasia and Dislocation
Stuart L. Weinstein
In the pediatric orthopaedic literature, the longstanding terminology of congenital dysplasia or congenital dislocation of the hip (CDH) has been progressively replaced by the term developmental dysplasia or developmental dislocation of the hip (DDH). (The term congenital dysplasia is attributed to Hippocrates; congenital implies that a condition existed at birth.) The American Academy of Orthopaedic Surgeons (1), the Pediatric Orthopaedic Society of North America, and the American Academy of Pediatrics have endorsed the name change of this entity from CDH to DDH, because the latter is more representative of the wide range of abnormalities seen in this condition (2).
The term developmental is more encompassing and is taken in the literal sense of organ growth and differentiation, including the embryonic, fetal, and infantile periods. This terminology includes all cases that are clearly congenital and those that are developmental, incorporating subluxation, dislocation, and dysplasia of the hip.
One of the most confusing areas in DDH is the terminology used in discussing the condition. What different investigators mean by “instability,” “dysplasia,” “subluxation,” and “dislocation” varies considerably. In this chapter, the term “DDH” denotes developmental dysplasia of the hip and encompasses all the variations of the condition described. Within this spectrum are two entities: dysplasia and dislocation. In the newborn, the term dysplasia refers to any hip with a positive Ortolani sign. This sign is the sensation the examiner feels upon provoking the femoral head into subluxation (i.e., partial contact between the femoral head and the acetabulum), dislocation (i.e., no contact between the femoral head and the acetabulum), or reduction from either of these positions. It is often difficult to make the distinction between these two entities, especially given the subtleties of arthrographic and ultrasonographic classifications. Because further subclassification in the newborn has no influence on treatment, the author prefers to use the term dysplasia to encompass these entities and other variations. In the newborn, the term dislocation refers here only to complete, irreducible dislocations.
NORMAL GROWTH AND DEVELOPMENT OF THE HIP JOINT
For the hip joint to grow and develop in a normal way, there must be a genetically determined balance of growth of the acetabular and triradiate cartilages and a well-located and centered femoral head. Embryologically, the components of
the hip joint, the acetabulum, and the femoral head develop from the same primitive mesenchymal cells (3, 4, 5 and 6) (Fig. 23-1). A cleft develops in the precartilaginous cells at about the seventh week of gestation. This cleft defines the acetabulum and the femoral head. By the 11th week of intrauterine life, the hip joint is fully formed (5, 6 and 7). Theoretically, the 11th week is the earliest time at which a dislocation could develop, although this rarely happens (7). Acetabular development continues throughout intrauterine life, particularly by means of growth and development of the labrum (3, 6). In the normal hip at birth, the femoral head is deeply seated in the acetabulum and held within the confines of the acetabulum by the surface tension of the synovial fluid. It is extremely difficult to dislocate a normal infant’s hip, even after incising the hip joint capsule (8, 9). The retaining force is similar to that of a suction cup. Hips in newborns with DDH are not merely normal hips with capsular laxity; they are pathologic entities.
the hip joint, the acetabulum, and the femoral head develop from the same primitive mesenchymal cells (3, 4, 5 and 6) (Fig. 23-1). A cleft develops in the precartilaginous cells at about the seventh week of gestation. This cleft defines the acetabulum and the femoral head. By the 11th week of intrauterine life, the hip joint is fully formed (5, 6 and 7). Theoretically, the 11th week is the earliest time at which a dislocation could develop, although this rarely happens (7). Acetabular development continues throughout intrauterine life, particularly by means of growth and development of the labrum (3, 6). In the normal hip at birth, the femoral head is deeply seated in the acetabulum and held within the confines of the acetabulum by the surface tension of the synovial fluid. It is extremely difficult to dislocate a normal infant’s hip, even after incising the hip joint capsule (8, 9). The retaining force is similar to that of a suction cup. Hips in newborns with DDH are not merely normal hips with capsular laxity; they are pathologic entities.
After birth, continued growth of the proximal femur and the acetabular cartilage complex is extremely important to the continuing development of the hip joint (3, 7, 10, 11, 12 and 13). The growth of these two members of the hip joint is interdependent.
Acetabular Growth and Development.
The acetabular cartilage complex (Fig. 23-2) is a three-dimensional structure that is triradiate medially and cup-shaped laterally. The acetabular cartilage complex is interposed between the ilium above, the ischium below, and the pubis anteriorly. Acetabular cartilage forms the outer two-thirds of the acetabular cavity, and the nonarticular medial wall of the acetabulum is formed by a portion of the ilium above, the ischium below, and portions of the triradiate cartilage.
Thick cartilage, from which a secondary ossification center, the os acetabulum (discussed later in this chapter), develops in early adolescence, separates the acetabular cavity from the pubic bone (11). The fibrocartilaginous labrum is at the margin of the acetabular cartilage, and the joint capsule inserts just above its rim (14) (Fig. 23-3).
The triradiate cartilage is a triphalangic structure. Each phalangis is composed of very cellular hyaline cartilage. This cartilage contains many canals. Each side of each limb of the triradiate cartilage has a growth plate. One phalangis is oriented horizontally between the ilium and the ischium. One phalangis is oriented vertically and interposed between the pubis and the ischium. The third phalangis is located anteriorly and slanted superiorly between the ilium and the pubis (Fig. 23-4). The triradiate cartilage is the common
physis of these three pelvic bones. Interstitial growth within the triradiate cartilage causes the hip joint to expand in diameter during growth (15).
physis of these three pelvic bones. Interstitial growth within the triradiate cartilage causes the hip joint to expand in diameter during growth (15).
The entire acetabular cartilage complex is composed of very cellular hyaline cartilage (Fig. 23-3). The lateral portion of the acetabular cartilage is homologous with other epiphyseal cartilages of the skeleton (16). This is important in understanding the normal growth and development and the shape of the acetabulum in skeletal dysplasias and injury. The labrum, or fibrocartilaginous edge of the acetabulum, is at the margin of the acetabular cartilage. The hip joint capsule inserts just above the labrum. The capsule insertion is continuous with the labrum below and with the periosteum of the pelvic bones above.
Articular cartilage covers the acetabular cartilage on the side that articulates with the femoral head. On the opposite side is a growth plate, with its degenerating cells facing toward the pelvic bone that it opposes. New bone formation occurs in the metaphysis adjacent to the degenerating cartilage cells. Growth of the acetabular cartilage occurs by means of interstitial growth within the cartilage and appositional growth under the perichondrium. This fact is most important when considering various innominate bone osteotomies, because surgical injury (by aggressive periosteal stripping or osteotome placement) to this important area may jeopardize further acetabular growth.
Growth of the Proximal Femur.
In the infant, the entire proximal end of the femur, including the greater trochanter, the intertrochanteric zone, and the proximal femur, is composed of cartilage. Between the fourth and seventh months of life, the proximal femoral ossification center appears. This bony centrum and its cartilaginous anlage continue to enlarge (although at a slowly decreasing rate) until adult life, at which stage only a thin layer of articular cartilage remains over it. The proximal femur and the trochanter enlarge by appositional cartilage cell proliferation (17).
The three main growth areas in the proximal femur are the physeal plate, the growth plate of the greater trochanter, and the femoral neck isthmus (17) (Fig. 23-5). A balance among the growth rates of these centers accounts for the normal configuration of the proximal femur, the relation between the proximal femur and the greater trochanter, and the overall width of the femoral neck. The growth of the proximal femur is affected by muscle pull, the forces transmitted across the hip joint by weight bearing, normal joint nutrition, circulation, and muscle tone (17, 18 and 19). Any alterations in these factors may cause profound changes in the development of the proximal femur (20, 21).
During infancy, a small cartilaginous isthmus connects the trochanteric and femoral growth plates along the lateral
border of the femoral neck and is a reflection of their previous common origin. This growth cartilage contributes to the lateral width of the femoral neck and remains active until maturity.
border of the femoral neck and is a reflection of their previous common origin. This growth cartilage contributes to the lateral width of the femoral neck and remains active until maturity.
It is the normal growth of these three physes that determines the femoral neck configuration in the adult. Disturbances in growth in any of these three growth plates, by whatever mechanism, alter the shape of the proximal femur. Hyperemia secondary to surgery or inflammatory conditions may stimulate growth in any or all of these growth plates (17).
The proximal femoral physeal plate contributes approximately 30% to the overall growth in length of the femur and 13% to the growth of the limb. Any damage to or disruption of the blood supply to this plate disrupts the growth at this plate and results in a varus deformity because the trochanter and the growth plate along the femoral neck continue to grow (17, 22). Partial physeal arrest patterns may be caused by damage to the portions of the proximal femoral physeal plate. The relation between the growth of the trochanter and the physis of the proximal femur should remain constant; it is measured by the articular trochanteric distance, which is the distance between the tip of the greater trochanter and the superior articular surface of the femoral head. The greater trochanter is usually classified as a traction epiphysis, depending on the normal abductor pull for growth stimulation. The trochanter, like the proximal femur, grows appositionally.
Determinants of Shape and Depth of the Acetabulum.
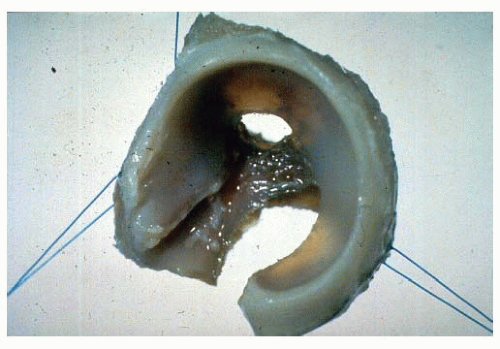
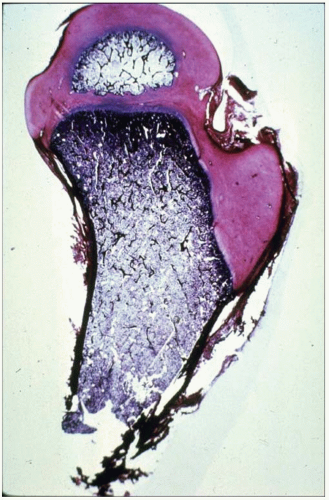
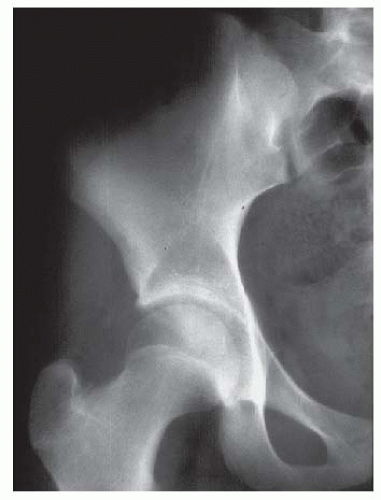
Experimental studies and clinical findings in humans with unreduced dislocations suggest that the main stimulus for the concave shape of the acetabulum is the presence of a spherical femoral head (10, 16, 23, 24 and 25). Harrison determined that the acetabulum failed to develop in area and depth after femoral head excision in rats (16). He also demonstrated atrophy and degeneration of the acetabular cartilage, although the growth plates of the triradiate cartilage remained histologically normal, as did the length of the innominate bones. These experimental findings are characteristic of humans who have had untreated hip dislocations (Fig. 23-6).
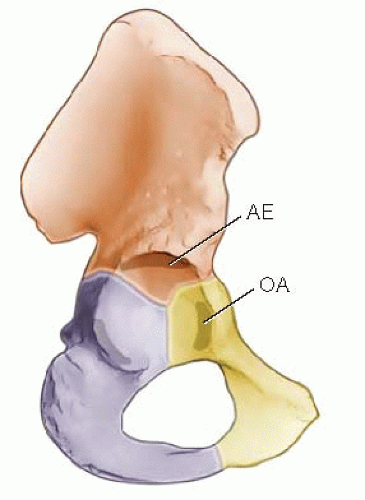
For the normal depth of the acetabulum to increase during development, several factors must act in concert. There must be a reduced spherical femoral head. There must also be normal interstitial and appositional growth within the acetabular cartilage, and periosteal new bone formation must occur in the adjacent pelvic bones (10, 11). The depth of the acetabulum is further enhanced at puberty by the development of three secondary centers of ossification (Fig. 23-7). These three centers are homologous with other epiphyses in the skeleton (11, 16). The os acetabulum develops in the thick cartilage that separates the acetabular cavity from the pubis. The os acetabulum is the epiphysis of the pubis and forms the anterior wall of the acetabulum. The epiphysis of the ilium, the acetabular epiphysis, forms a major portion of the superior edge of the acetabulum. A third, small epiphysis also forms in the ischial region and contributes to its normal growth (11, 16, 26).
Normal acetabular growth and development occur through balanced growth of the proximal femur, the acetabular
and triradiate cartilages, and the adjacent bones. This balance, which is probably genetically determined, may be faulty in DDH. There is ample evidence to suggest that an adverse intrauterine environment also plays an important role in the pathogenesis of hip dysplasia (11, 27, 28, 29, 30 and 31).
and triradiate cartilages, and the adjacent bones. This balance, which is probably genetically determined, may be faulty in DDH. There is ample evidence to suggest that an adverse intrauterine environment also plays an important role in the pathogenesis of hip dysplasia (11, 27, 28, 29, 30 and 31).
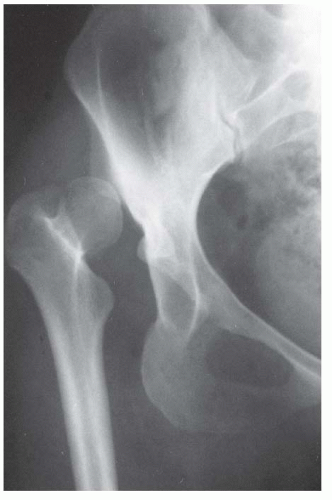 FIGURE 23-6. Untreated dislocation of the hip. Note the lack of the concave shape and the shallowness of the acetabulum. |
PATHOANATOMY
Dislocations in Newborns.
In the newborn with DDH, the tight fit between the femoral head and the acetabulum is lost. The femoral head can be made to glide in and out of the acetabulum, with a palpable sensation known clinically as the Ortolani sign (11, 19, 32, 33). DDH in the newborn refers to a spectrum of anatomic abnormalities, from mild dysplastic changes to the severe pathoanatomic changes, that are found in the rare idiopathic teratologic dislocation and more commonly in teratologic dislocations associated with conditions such as myelomeningocele and arthrogryposis.
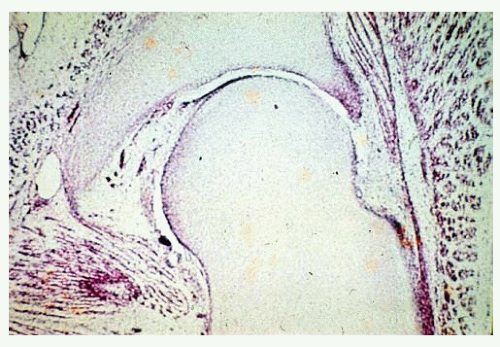
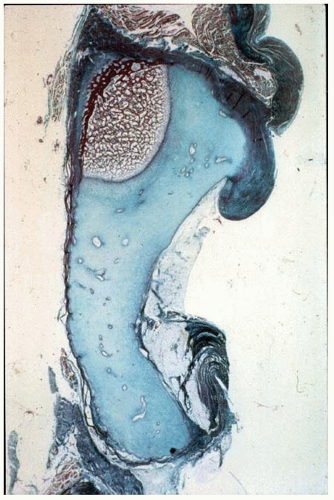
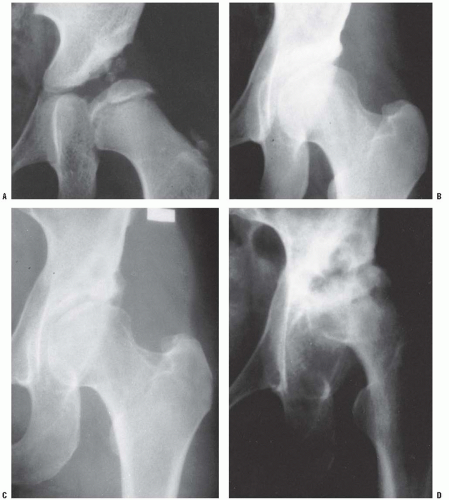
The most common pathologic change in the newborn with DDH is a hypertrophied ridge of acetabular cartilage in the superior, posterior, and inferior aspects of the acetabulum. This ridge was referred to by Ortolani as the neolimbus (19, 33). The neolimbus is composed of hypertrophied acetabular cartilage (9, 32) (Fig. 23-8). There often is a trough or groove in the acetabular cartilage caused by secondary pressure of the femoral head or neck. It is over this ridge of acetabular cartilage that the femoral head glides in and out of the acetabulum, with the palpable sensation referred to as the Ortolani sign (9, 19, 33).
There is empiric evidence that the pathologic changes are reversible in the typical newborn with DDH, because there is a 95% success rate of treatment using simple devices such as the Pavlik harness and the von Rosen splint (34). These pathologic changes are typical of 98% of DDH cases that occur at or around birth. However, approximately 2% of newborns have teratologic (antenatal) dislocations not associated with a syndrome or neuromuscular condition (26, 29). In these rare cases, the pathologic and clinical findings are similar to those seen in late-diagnosed DDH, which is described later in this chapter.
Acetabular Development in Developmental Hip Dysplasia.
Acetabular development in treated DDH cases may be different from that described for the normal hip. This is particularly true for late-diagnosed cases. The primary stimulus for normal growth and development comes from the femoral head within the acetabulum (10, 24, 25). When there is a delay in diagnosis and treatment, some aspects of normal growth and development are lost. The femoral head must be reduced as soon as possible, and the reduction must be maintained to provide the stimulus for acetabular development. If concentric reduction is maintained, the acetabulum has the potential for recovery and resumption of normal growth and development for many years (35, 36 and 37).
The age at which a dysplastic hip can still return to “normal” after reduction remains controversial (34, 36, 37, 38, 39, 40, 41, 42, 43 and 44).
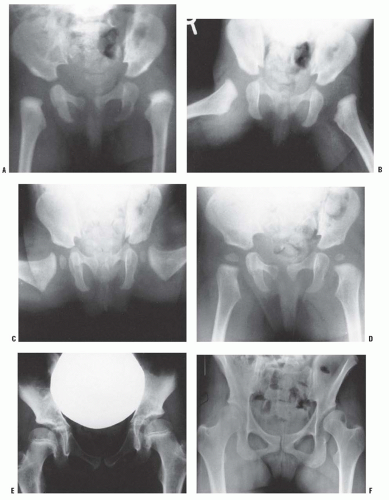
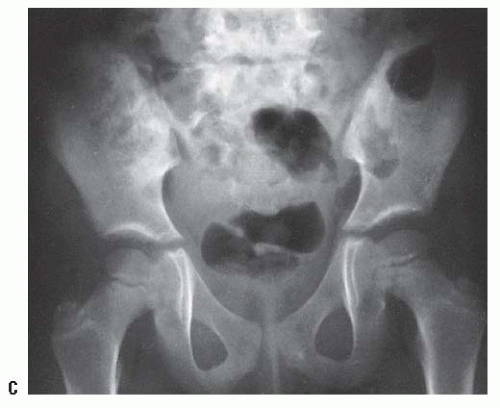
The resumption and adequacy of acetabular development is a multifactorial problem that depends on the age at which the reduction is obtained and on whether the growth potential of the acetabular cartilage and the proximal femur is normal. The capacity of the acetabular cartilage to resume normal growth depends on its intrinsic growth potential and whether it has been damaged by the subluxated or dislocated femoral head or by various attempts at reduction. In the patient with DDH who has been treated, especially in late-diagnosed cases, accessory centers of ossification contribute to acetabular development (Fig. 23-9). Accessory centers of ossification in the acetabulum are seen in only 2% to 3% of normal hips, and they rarely appear before 11 years of age. However, among patients treated for DDH, the centers may be present in as many as 60% of hips, usually appearing 6 months to 10 years after reduction (35, 36, 37 and 38, 45) (Fig. 23-9). These accessory centers form in the peripheral acetabular cartilage and may be a primary abnormality of dysplasia or, more likely, they are a secondary abnormality caused by pressure damage from the femoral head and/or neck in the subluxated or dislocated position or by damage secondary to closed or open treatment (see later discussion on obstacles to reduction). In treated DDH cases, these accessory centers of ossification should be sought on every sequential radiograph so as to determine whether acetabular development is progressing, as they may coalesce to form a normal acetabulum (Fig. 23-9). This is an important factor to consider when deciding if surgical intervention is necessary to correct residual acetabular dysplasia. Although the presence of these centers indicates continued growth in the acetabular cartilage, they may be indicative of injury to the cartilage in this area. Their presence does not assure normal acetabular development.
The resumption and adequacy of acetabular development is a multifactorial problem that depends on the age at which the reduction is obtained and on whether the growth potential of the acetabular cartilage and the proximal femur is normal. The capacity of the acetabular cartilage to resume normal growth depends on its intrinsic growth potential and whether it has been damaged by the subluxated or dislocated femoral head or by various attempts at reduction. In the patient with DDH who has been treated, especially in late-diagnosed cases, accessory centers of ossification contribute to acetabular development (Fig. 23-9). Accessory centers of ossification in the acetabulum are seen in only 2% to 3% of normal hips, and they rarely appear before 11 years of age. However, among patients treated for DDH, the centers may be present in as many as 60% of hips, usually appearing 6 months to 10 years after reduction (35, 36, 37 and 38, 45) (Fig. 23-9). These accessory centers form in the peripheral acetabular cartilage and may be a primary abnormality of dysplasia or, more likely, they are a secondary abnormality caused by pressure damage from the femoral head and/or neck in the subluxated or dislocated position or by damage secondary to closed or open treatment (see later discussion on obstacles to reduction). In treated DDH cases, these accessory centers of ossification should be sought on every sequential radiograph so as to determine whether acetabular development is progressing, as they may coalesce to form a normal acetabulum (Fig. 23-9). This is an important factor to consider when deciding if surgical intervention is necessary to correct residual acetabular dysplasia. Although the presence of these centers indicates continued growth in the acetabular cartilage, they may be indicative of injury to the cartilage in this area. Their presence does not assure normal acetabular development.
PATHOGENESIS, EPIDEMIOLOGY, AND DIAGNOSIS
Causes of Developmental Dysplasia of the Hip.
Many factors contribute to DDH. Genetic and ethnic factors play a key role, with the incidence of DDH as high as 25 to 50 in 1,000 live births among Lapps and Native Americans and a very low rate among the southern Chinese population and persons of African descent (30, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 and 60).
A positive family history for DDH may be found in 12% to 33% of patients who have DDH (31, 46, 60). One study reported a tenfold increase in the incidence of DDH among the parents of index patients and a sevenfold increase among siblings compared with the incidence among the general population (46). There is some suggestion that anteversion of the femoral neck or acetabulum may be an etiologic factor (30, 32, 38, 61, 62 and 63).
The genetic effects on the hip joint in patients with DDH are revealed as primary acetabular dysplasia, various degrees of joint laxity, or a combination of both. Intrauterine mechanical factors, such as breech position or oligohydramnios, and neuromuscular mechanisms, such as myelomeningocele, can profoundly influence genetically determined intrauterine growth (5, 6, 64, 65). The first-born child is more likely to be affected than subsequent children. Any of the factors contributing to an “adverse” intrauterine environment may influence the development of the hip joint, and postnatal influences may also contribute to the development of DDH (5, 31, 66, 67, 68 and 69).
Risk Factors and Incidence.
Whites show an increased incidence of DDH among first-born children (8, 27, 28, 70, 71, 72 and 73). The unstretched abdominal muscles and the primigravida uterus may subject the fetus to prolonged periods of abnormal positioning, forcing the fetus against the mother’s spine. This restraint limits fetal mobility, especially hip abduction. The high rate of association of DDH with other intrauterine molding abnormalities, such as torticollis and metatarsus adductus, lends some support to the theory that the “crowding phenomenon” plays a role in the pathogenesis (8, 73, 74 and 75). Oligohydramnios, which is associated with limited fetal mobility, also is associated with DDH (8, 73). The left hip is the most commonly affected hip; in the most common fetal position, this is the hip that is usually forced into adduction against the mother’s sacrum (8, 48, 73).
DDH is more common among girls (80% of cases) and among children delivered in the breech presentation. In the general population, breech presentations occur in approximately 2% to 4% of vaginal deliveries. Carter and Wilkinson (27, 28) reported that 17% of children with DDH had a breech presentation; Salter reported the incidence as 23% (76). Twice as many girls as boys are born breech (77). Fiftynine percent of breech presentations are first-born children (27, 28, 77). Ramsey and MacEwen demonstrated that 1 of 15 girls born breech has evidence of hip instability. In animal studies, the prolonged maintenance of an abnormal position, such as the breech position, is associated with the production of DDH (24, 25).
The postnatal environment may significantly influence the development of DDH. In societies that use swaddling (i.e., hips forced into adduction and extension) in the immediate postnatal period, the incidence of DDH is high, possibly as a result of the forceful positioning of the legs in extension and adduction, counter to normal newborn hip flexion and hamstring contractures (31, 48, 58, 63, 68, 78, 79, 80 and 81).
The influence of hip capsular laxity on the development of DDH has been addressed by many investigators. Newborns with DDH may have capsular laxity. Hip capsular laxity has been implicated in the pathogenesis of DDH, because the diagnostic test for DDH, the Ortolani sign, depends on the head gliding in and out of the dysplastic acetabulum over a ridge of abnormal acetabular cartilage. Proponents argue that because reversible dysplasia can be produced in animals by producing ligamentous laxity, the acetabular dysplasia seen in DDH is a secondary phenomenon (24, 25, 31, 32, 76, 82). LeDamany demonstrated that the acetabulum is shallowest at birth (62).
Ralis and McKibbin confirmed LeDamany’s anatomic work in a small number of patients (82). They too demonstrated that the acetabulum was shallowest at birth and that this, combined with the normal laxity in the joint of the infant, makes the time around delivery a high-risk period for dislocation (82, 83). These anatomic experiments were repeated by Skirving and Scadden in African neonates (30); in the African neonate, the acetabulum was deeper more frequently and in a narrower range, possibly explaining why DDH is almost nonexistent among persons of African descent. This finding also provides indirect evidence that acetabular dysplasia is a primary cause of DDH.
Ralis and McKibbin confirmed LeDamany’s anatomic work in a small number of patients (82). They too demonstrated that the acetabulum was shallowest at birth and that this, combined with the normal laxity in the joint of the infant, makes the time around delivery a high-risk period for dislocation (82, 83). These anatomic experiments were repeated by Skirving and Scadden in African neonates (30); in the African neonate, the acetabulum was deeper more frequently and in a narrower range, possibly explaining why DDH is almost nonexistent among persons of African descent. This finding also provides indirect evidence that acetabular dysplasia is a primary cause of DDH.
Laxity of the hip joint capsule is often seen in newborn infants and has been documented by ultrasonography (84). The laxity may allow some instability without a positive Ortolani sign. In postmortem examination of seven stillborn infants, the hips demonstrated instability with a negative Ortolani sign; arthrograms demonstrated slight pooling of the contrast media medially. On gross examination, the hip capsules were stretched, and the femoral heads could be pulled slightly away from the acetabula. However, the hips were anatomically and histologically normal, unlike the postmortem findings reported for all infants with positive Ortolani signs (9, 11, 33, 56, 85, 86). In addition to the normal physiologic capsular laxity expected in the newborn, DDH is not a feature of conditions characterized by hyperlaxity, such as Down, Ehlers-Danlos, and Marfan syndromes (33).
Taking into account the epidemiologic and etiologic factors, a high-risk group of patients can be identified. This group includes any patient who has more than one of the factors listed in Table 23-1. If an infant manifests any combination of these factors, the physician should be alert to the possibility of DDH.
The incidence of DDH is influenced by geographic and ethnic factors and by the diagnostic criteria used by the examining physician. Another important factor is the diagnostic acumen of the examiner. The age of the patient at the time of diagnosis must be taken into account, because the physical findings and manifestations of the condition change with increasing delay in diagnosis (8, 9, 11, 31, 33, 56, 73, 84, 85, 86, 87, 88, 89 and 90).
TABLE 23-1 High-Risk Factors for Developmental Dysplasia or Dislocation of the Hip | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Most patients with DDH are detectable at birth (91, 92). Despite screening programs for newborns, some cases are not detected, and some evidence suggests that a few cases may arise after birth (30, 87, 92, 93, 94, 95, 96, 97, 98, 99, 100 and 101). Moreover, whether acetabular dysplasia is primary or secondary to an unrecognized dislocation or subluxation that has reduced spontaneously remains uncertain.
Diagnosis.
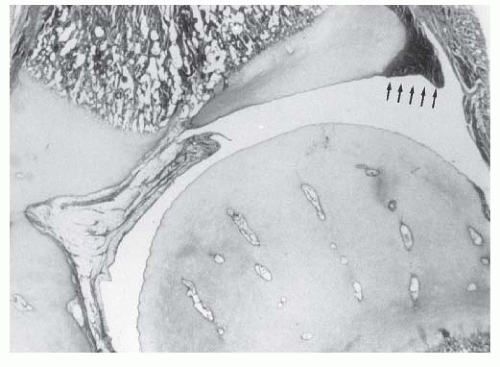
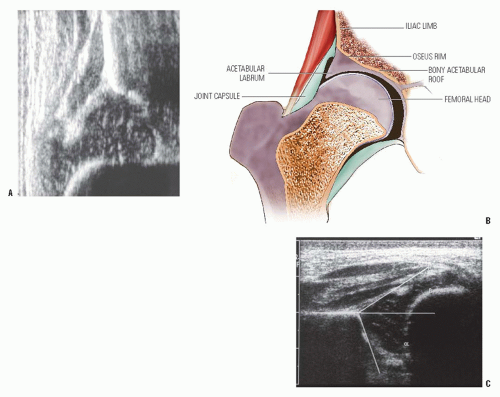
The clinical diagnostic test for DDH was originally described by LeDamany in 1912 (62). LeDamany referred to the palpable sensation of the hip gliding in and out of the acetabulum as the signe de ressaut. In 1936, Ortolani, an Italian pediatrician, described the pathogenesis of this diagnostic sign (33, 86). Ortolani called the palpable sensation the segno dello scotto. Fellander et al. likened this diagnostic sign to the femoral head gliding in and out of the acetabulum over a ridge and referred to this palpable sensation as the ridge phenomenon (102). This ridge, over which the femoral head glides in and out of the acetabulum, is composed of hypertrophied acetabular cartilage (9, 32, 33, 86) (Fig. 23-8). Ortolani named the ridge the neolimbus.
Unfortunately, inadequate translation of LeDamany’s and Ortolani’s works into English resulted in the use of the term click to describe this diagnostic sign. High-pitched soft-tissue clicks are often elicited in the hip examination of newborns. These clicks are usually transmitted from the trochanteric region or the knee and have no diagnostic significance (102). This poor understanding of the pathoanatomy of the primary diagnostic sign of DDH in the newborn has no doubt led to overdiagnosis and overtreatment of infants (103, 104, 105 and 106).
Another diagnostic test, the Barlow maneuver, is often referred to as the “click of exit.” The Barlow maneuver is a provocative maneuver in which the hip is flexed and adducted and the femoral head is palpated to exit the acetabulum partially or completely over a ridge of the acetabulum (87). Many physicians refer to the Ortolani sign as the click of entry, which is caused when the hip is abducted, the trochanter is elevated, and the femoral head glides back into the acetabulum. Some physicians make treatment decisions on the basis of whether they feel that the hip is Ortolani positive rather than Barlow positive, the general opinion being that the Barlow-positive hip is more stable and hence may stabilize spontaneously. Because Ortolani and LeDamany described the palpable sensation as the femoral head exits or enters the acetabulum, the author prefers to use the Ortolani sign to refer to both the palpable sensation of subluxating or dislocating the hip and to reducing a subluxated or dislocated hip. The author also makes no distinction in treatment between patients exhibiting the Ortolani sign and those with the Barlow sign.
Newborn clinical screening programs estimate that 1 of every 100 newborns examined has evidence of some hip instability (i.e., positive Ortolani or Barlow sign), although the incidence of true dislocation is reported to be between 1 and 1.5 cases per 1,000 live births (9, 44, 87, 95, 96, 97 and 98, 103, 107, 108, 109, 110, 111 and 112).
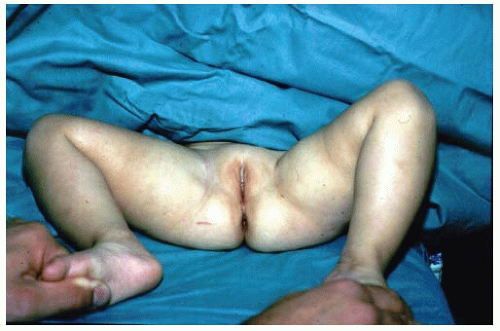 FIGURE 23-10. A 15-month-old child with left hip dislocation. Note the limited abduction of the hip. |
Complete irreducible dislocations are extremely rare in newborns and are usually associated with other generalized conditions, such as arthrogryposis, myelodysplasia, and other syndromes. These perinatal teratologic dislocations are at the extreme end of the DDH pathologic spectrum and account for only 2% of cases in newborn examination series (9, 48, 49, 113, 114). They are usually manifested because of the secondary adaptive changes more characteristic of the late-diagnosed case.
Late Diagnosis.
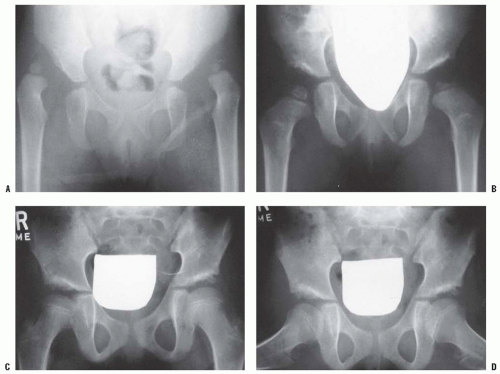
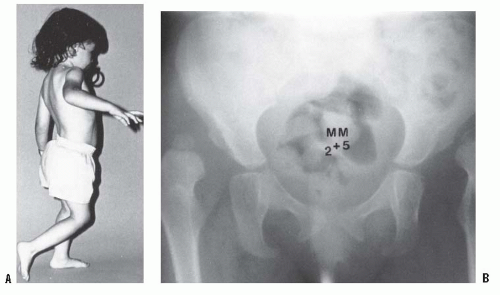
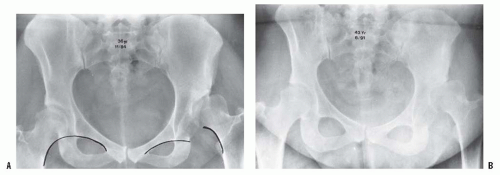
If the diagnosis of DDH is not made early, preferably in the newborn nursery, secondary adaptive changes develop (47). The most reliable physical finding in late-diagnosed DDH is limitation of abduction (Fig. 23-10). Limited abduction is a clinical manifestation of the various degrees of shortening of the adductor longus that are associated with hip subluxation or dislocation (49). Other manifestations of late-diagnosed DDH may include apparent femoral shortening, also called the Galeazzi sign (Fig. 23-11); asymmetry of the gluteal (115), thigh, or labial folds (116); and limb-length inequality (Fig. 23-12). In patients with bilateral dislocations, clinical findings include a waddling gait and hyperlordosis of the lumbar spine (Fig. 23-13A).
If DDH goes undetected, normal hip joint growth and development are impaired. With increasing age at detection and reduction, and particularly in children older than 6 months, the obstacles (intraarticular and extraarticular) to concentric reduction become increasingly difficult to overcome by simple treatment methods such as use of the Pavlik harness, and closed or open reduction usually must be performed under general anesthesia. Restoration of normal acetabular development is less likely as age at detection increases (26, 32, 44, 111, 115, 116, 117, 118 and 119).
In the late-diagnosed case, the extraarticular obstacles to reduction include the contracted adductor longus and the iliopsoas. These muscles are shortened because of the hip being in the subluxated or dislocated position, allowing secondary muscle shortening.
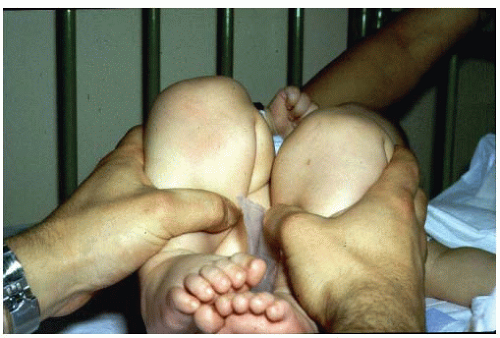 FIGURE 23-11. A 15-month-old girl with developmental dislocation of the left hip. Note the apparent femoral shortening. |
The intraarticular obstacles to reduction in late-diagnosed DDH include the ligamentum teres, the transverse acetabular ligament, the constricted anteromedial joint capsule, and, rarely, an inverted and hypertrophied labrum (32, 120). The most significant intraarticular obstacle to reduction, however,
is some degree of anteromedial hip capsular constriction (32, 121, 122, 123, 124 and 125). The ligamentum teres may be thickened, and it may become the primary obstacle to reduction in some cases. In children of walking or crawling age, the ligamentum teres may be significantly elongated and enlarged. Its sheer bulk precludes concentric reduction without excision of the ligament. The transverse acetabular ligamentum may hypertrophy secondary to the constant pull of the ligamentum teres on its attachment at the base of the acetabulum (32, 125). This effect decreases the diameter of the acetabulum.
is some degree of anteromedial hip capsular constriction (32, 121, 122, 123, 124 and 125). The ligamentum teres may be thickened, and it may become the primary obstacle to reduction in some cases. In children of walking or crawling age, the ligamentum teres may be significantly elongated and enlarged. Its sheer bulk precludes concentric reduction without excision of the ligament. The transverse acetabular ligamentum may hypertrophy secondary to the constant pull of the ligamentum teres on its attachment at the base of the acetabulum (32, 125). This effect decreases the diameter of the acetabulum.
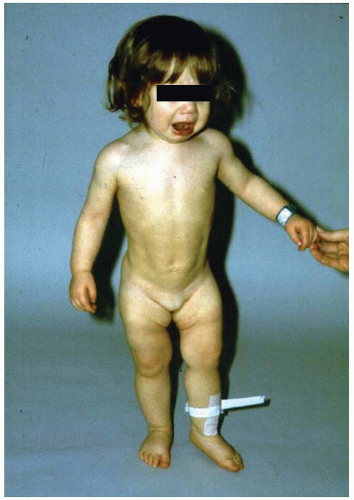 FIGURE 23-12. A 1-year-old girl with developmental dislocation of the left hip was referred for toe walking. Note the apparent limblength inequality and the asymmetry of the thigh and labial folds. |
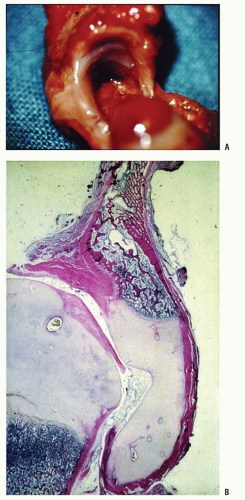
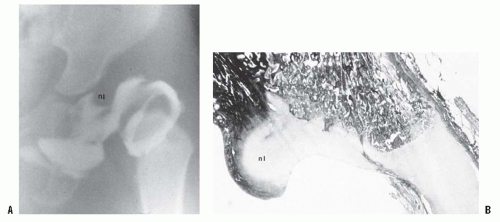
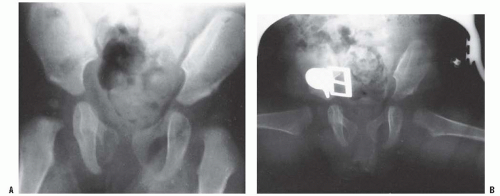
A rare finding, other than in teratologic dislocations, is a true inverted labrum or limbus (i.e., hypertrophied labrum) (122) (Fig. 23-14). The acetabular labrum may be iatrogenically inverted and may be an obstacle to reduction in patients previously treated with unsuccessful closed reductions. Arthrograms are often misinterpreted as showing an inverted labrum (126); the shadow thought to be the inverted labrum or limbus is instead the neolimbus (originally described by Ortolani) (9, 32, 127, 128) (Fig. 23-15). This neolimbus is epiphyseal cartilage and is almost never an obstacle to reduction. It must not be removed, because removal impairs acetabular development (9, 129). If the surgeon feels that this tissue is somehow impairing reduction, it should be radially incised but never excised. The cartilage of the neolimbus may be primarily abnormal or may be damaged by a traumatic open or closed reduction. A response to this damage may be responsible for the appearance of the previously discussed accessory centers of ossification seen in treated cases of DDH (9) (Fig. 23-9).
Diagnostic Imaging and Radiography.
Although the clinical examination remains the gold standard (130), ultrasonography has gained popularity worldwide as a screening tool. Its cost-effectiveness has yet to be documented for screening for DDH on a wide scale.
The use of ultrasonography in the diagnosis and management of children with DDH remains controversial (131, 132, 133, 134, 135, 136, 137, 138, 139, 140 and 141).
Many proponents strongly recommend that ultrasonography be used as a routine screening tool in the newborn nursery and that it be used extensively in the management of all DDH problems (142, 143 and 144). The use of ultrasonography in orthopaedic practice was pioneered by Graf in Austria in the 1970s (145, 146). Harcke et al. in the United States (147, 148, 149 and 150), Terjesen et al. in Norway (151, 152, 153 and 154), and Clarke in Great Britain (155, 156 and 157) have been the prime evaluators of this tool for the diagnosis of DDH and other hip disorders.
Many proponents strongly recommend that ultrasonography be used as a routine screening tool in the newborn nursery and that it be used extensively in the management of all DDH problems (142, 143 and 144). The use of ultrasonography in orthopaedic practice was pioneered by Graf in Austria in the 1970s (145, 146). Harcke et al. in the United States (147, 148, 149 and 150), Terjesen et al. in Norway (151, 152, 153 and 154), and Clarke in Great Britain (155, 156 and 157) have been the prime evaluators of this tool for the diagnosis of DDH and other hip disorders.
Ultrasonography can be used in two basic ways to evaluate the child with DDH: morphologic assessment and dynamic assessment (158, 159, 160, 161, 162 and 163). The morphologic assessment, as pioneered by Graf, focuses primarily on critical evaluation of the anatomic characteristics of the hip joint (Fig. 23-16). This is accomplished by measuring two angles on the ultrasound image: the α angle, which is a measurement of the slope of the superior aspect of the bony acetabulum, and the β angle, which evaluates the cartilaginous component of the acetabulum. The hip is classified into four types and several subtypes according to various factors (145, 146) (Fig. 23-17). In the evaluation of Terjesen et al., the percentage of acetabular coverage of the femoral head (i.e., percent coverage) is a key measurement (151, 152, 153 and 154).
The morphologic approach to ultrasonography is widely practiced in Europe, but it has been criticized because of substantial interobserver and intraobserver variations in the measurement of angles, particularly the β angle (148).
The availability of equipment with which motion can be observed in real time and in multiple planes provides a means of seeing what occurs during the Ortolani or Barlow maneuver. The use of dynamic ultrasonography, as popularized by Harcke et al. (147, 148 and 149), has been criticized for being excessively operator dependent and requiring a subjective assessment of the findings.
The indications for ultrasonography in the diagnosis and treatment of DDH are not universally established. Because there are many controversies yet to be resolved, ultrasonography cannot be advocated as a routine screening tool, even though (148) it is used as such in Europe and in many centers around the world. Prospective longitudinal studies documenting the outcomes of minor anatomic abnormalities found in ultrasonographic examinations need to be completed (164, 165). Its routine use in newborn nurseries has resulted in overdiagnosis (above the expected incidence) of DDH and cannot be considered cost-effective (166, 167, 168 and 169). Its use in only high-risk infants may eventually prove cost-effective (169, 170 and 171). However, Clarke et al. showed that screening all high-risk infants and all infants who had any abnormality on physical examination did not reduce the prevalence of latediagnosed cases (156, 157, 172).
Some centers advocate the use of ultrasonography in all Ortolani-positive infants to assess stability at the completion of treatment (148). An ideal use for ultrasonography is for “guided reduction” of a dislocated hip in an infant (173), in other words, for monitoring the progress of reduction of a subluxated or dislocated hip being treated in a Pavlik harness. Ultrasonography is used at 7- to 10-day intervals to check the progress of reduction of the hip and its stability during Pavlik harness treatment. This may temporarily obviate the need for radiographic evaluation. Other uses for ultrasonography in the treatment of DDH include monitoring of the hip position while the patient is in traction before attempting reduction and evaluating closed reductions in the operating room. The distinct advantage of ultrasonography is that it provides some anatomic evaluation of the hip joint without exposing the infant to radiation.
Debate continues regarding the appropriate planes for evaluation and whether an orthopaedic surgeon, the treating physician, or a radiologist with expertise in ultrasonography
should perform the evaluation. In the newborn, DDH is not a radiographic diagnosis; the diagnosis should be made by clinical evaluation, which may be enhanced by ultrasonography if the examination results are questionable. Some investigators have used vibration arthrometry (174, 175 and 176).
should perform the evaluation. In the newborn, DDH is not a radiographic diagnosis; the diagnosis should be made by clinical evaluation, which may be enhanced by ultrasonography if the examination results are questionable. Some investigators have used vibration arthrometry (174, 175 and 176).
After the newborn period (4 to 6 weeks of age), the diagnosis of DDH should be confirmed by radiography. Many radiographic measurements can be made, but there are wide interobserver and intraobserver variations in these measurements (177, 178). Because it is difficult to standardize the radiographic positioning of infants, many centers use positioning frames (179).
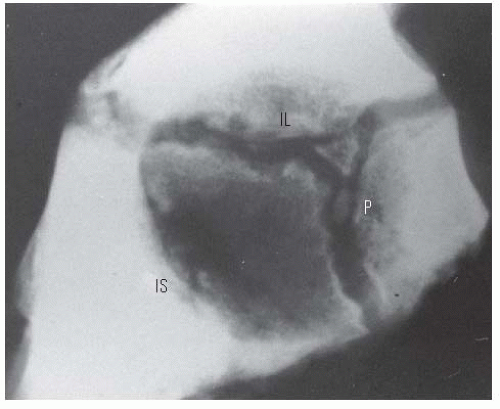
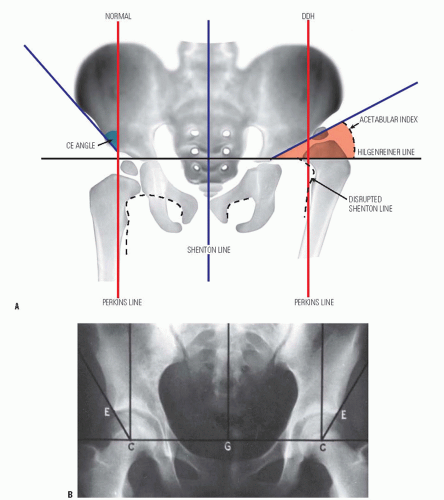
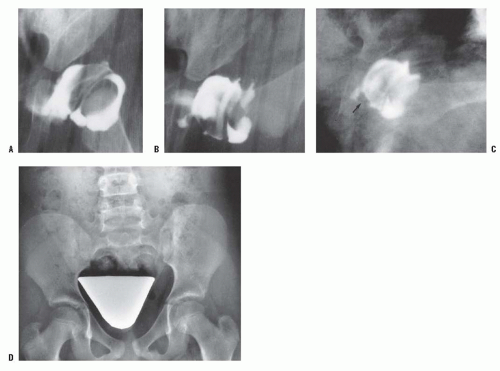
When monitoring the treatment of children with DDH, it is essential to notice changes in the radiographic measurements over time and not to make significant decisions based on a single radiograph. The classic radiographic features of late-diagnosed DDH include an increased acetabular index (29, 180, 181, 182, 183, 184 and 185), disruption of the Shenton line, a widened pelvic floor (186, 187), an absent teardrop figure (188, 189, 190, 191, 192, 193 and 194), delayed appearance of the femoral ossific nucleus on the involved side or dissimilar sizes of the ossific nuclei, abnormality in Smith centering ratios (24, 25, 37), decreased femoral head coverage, and failure of the medial metaphyseal beak of the proximal femur, and, subsequently, the secondary ossification center, to be located in the lower inner quadrant defined by the Hilgenreiner and Perkins lines (89, 195, 196) (Fig. 23-18A). When the triradiate cartilage is closed, the acetabular angle of Sharp (i.e., from the inferior edge of the teardrop figure to the edge of the acetabulum) is a useful measurement of acetabular dysplasia (197).
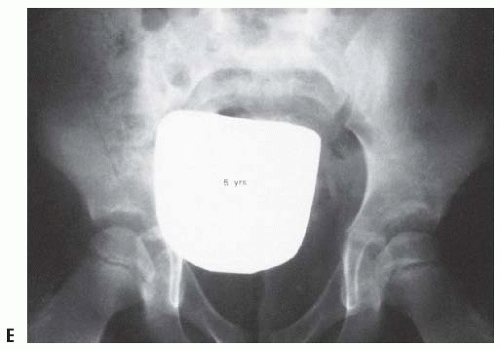
In children younger than 8 years, the acetabular index is a reasonable measure of acetabular development (177). Measurement of the center-edge (CE) angle becomes useful only in the patient who is more than 5 years of age and is most useful in the adult patient (45) (Fig. 23-18B). Radiographs show only the ossified portion of the pelvic bones and the proximal femur. Excellent acetabular coverage of the femoral head may be found, albeit by unossified cartilage (Fig. 23-19). If this cartilage does not ossify, the residual dysplasia may eventually lead to subluxation and degenerative joint disease.
The Shenton line provides only a qualitative estimate of dysplasia during the first few years of life. After 3 or
4 years of age, the Shenton line should be intact on all views of the hip; thereafter, any disruption of the Shenton line indicates an abnormality in the relation between the proximal femur and the acetabulum. As will be emphasized in the section on methods of treatment, this relation must be restored in order to prevent degenerative joint disease in later life (26, 45, 58, 63, 118).
4 years of age, the Shenton line should be intact on all views of the hip; thereafter, any disruption of the Shenton line indicates an abnormality in the relation between the proximal femur and the acetabulum. As will be emphasized in the section on methods of treatment, this relation must be restored in order to prevent degenerative joint disease in later life (26, 45, 58, 63, 118).
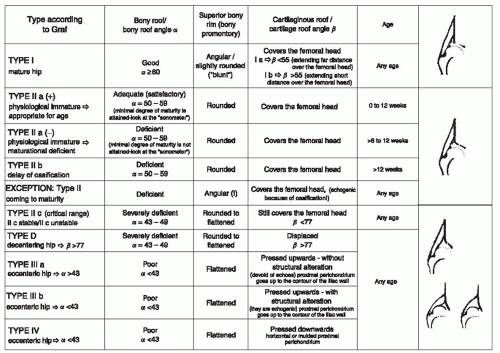 FIGURE 23-17. Hip types based on ultrasonographic results. (Courtesy of Prof. R. Graf, Stolzalpe, Austria.) |
Magnetic resonance imaging (MRI) has been used for DDH diagnosis and evaluation, as well as for documentation of femoral head-acetabular relationships after closed or open reduction (198, 199). With advances in software, this modality will no doubt provide useful information in the future, but the need for anesthesia in infants and children limits its utility (200, 201, 202 and 203).
NATURAL HISTORY
Course in Newborns.
The natural history of untreated DDH in the newborn is quite variable. Yamamuro and Doi followed up 52 patients whose hips had positive Ortolani signs for a 2-year period without treatment for the first 5 months. Of the 12 that they called “dislocated” hips, three (25%) were radiographically normal at 5 months of age. Of the 42 that they called “subluxable” hips, 24 (57%) were normal at 5 months (81).
Barlow reported that 1 of every 60 infants born has instability (i.e., positive Barlow sign) of one or both hips (87). More than 60% of these stabilize during the first week of life and 88% stabilize during the first 2 months without treatment. The remaining 12% become true congenital dislocations and persist in the absence of treatment. Pratt et al. did a follow-up, for an average of 11.2 years, of 18 “dysplastic” hips in patients who had been diagnosed on the basis of clinical and radiographic parameters at an age younger than 3 months. They found that 15 of these hips were radiographically normal (80).
Coleman followed up 23 untreated patients who had been diagnosed as having DDH on the basis of clinical and radiographic criteria at younger than 3 months. He found that 26% of the femoral heads became completely dislocated, 13% had partial contact of the femoral head with the acetabulum, 39% remained located but retained dysplastic features, and 22% were normal (48).
Most unstable hips in newborns stabilize soon after birth, some may go on to subluxation (partial contact of femoral head
with the acetabulum) or dislocation, and some may remain located (intact Shenton line) but retain anatomic dysplastic features. Because it is not possible to predict the outcome of unstable hips in newborns, all newborns with clinical hip instability, as manifested by a positive Ortolani or Barlow sign, should be treated.
with the acetabulum) or dislocation, and some may remain located (intact Shenton line) but retain anatomic dysplastic features. Because it is not possible to predict the outcome of unstable hips in newborns, all newborns with clinical hip instability, as manifested by a positive Ortolani or Barlow sign, should be treated.
Course in Adults.
In adults, the natural history of untreated complete dislocation varies and is affected by societal considerations (57, 66, 198, 199, 200 and 201). Despite complete dislocation, there may be little or no functional disability.
The natural history of complete dislocation depends on the presence or absence of two factors: a well-developed false acetabulum and bilaterality (27, 58, 67, 117, 204, 205). Wedge and Wasylenko demonstrated only a 24% chance of a good clinical outcome with a well-developed false acetabulum, but with a moderately developed or absent false acetabulum, the patients had a 52% chance of a good clinical outcome
(58, 67). Of 42 patients with complete dislocations, 13 had radiographically confirmed degenerative joint disease, such as loss of joint space, cyst formation, sclerosis, osteophyte formation, and flattening of the femoral head. Of these 13 patients, 10 (76%) had poor clinical outcomes.
(58, 67). Of 42 patients with complete dislocations, 13 had radiographically confirmed degenerative joint disease, such as loss of joint space, cyst formation, sclerosis, osteophyte formation, and flattening of the femoral head. Of these 13 patients, 10 (76%) had poor clinical outcomes.
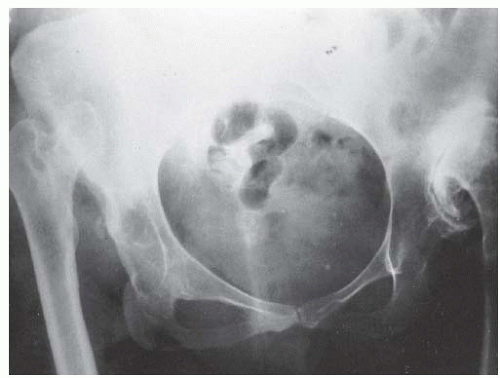
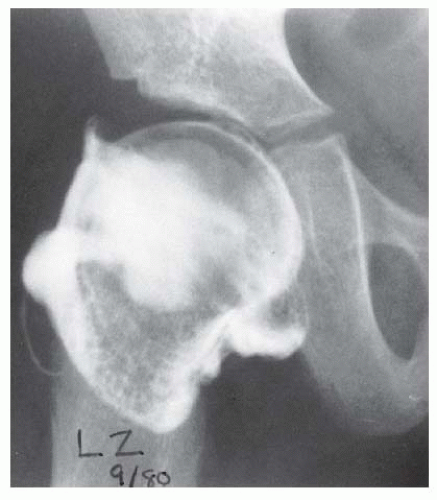 FIGURE 23-19. Arthrogram of a 5-year-old girl 3 years after open reduction. Note the excellent coverage of the femoral head by unossified acetabular cartilage. |
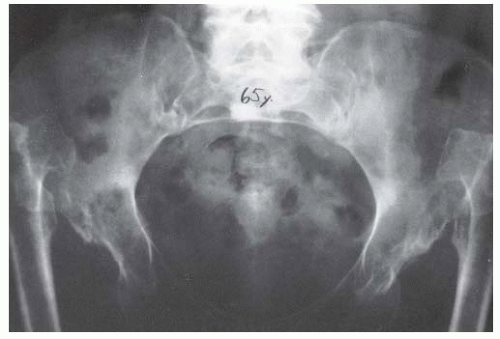
Milgram reported the gross and histologic features of a case of bilateral DDH discovered at postmortem examination (206). This 74-year-old man had no hip or thigh pain and only mild backache for 5 years before his death. The femoral head had no articulation with any portion of the ilium and was covered with a thickened, markedly elongated hip joint capsule. The only degenerative changes were where the lesser trochanter abutted the overhanging superior acetabular rim. In the absence of a false acetabulum, most patients with complete dislocations do well, maintaining good range of motion with little functional disability (Fig. 23-20). Completely dislocated hips with well-developed false acetabula are more likely to develop radiographically visible degenerative joint disease changes and poor clinical outcomes (Fig. 23-21). Factors that lead to the formation or lack of formation of a false acetabulum remain unknown (26).
Back pain may occur in patients with bilateral dislocations. It is thought that this pain is secondary to the hyperlordosis of the lumbar spine that is associated with bilateral dislocations (26, 58, 67, 206, 207 and 208) (Melvin P, Johnston R, Ponseti IV, personal communication) (Fig. 23-22).
In unilateral complete dislocations, secondary problems of limb-length inequality, ipsilateral knee deformity and pain, scoliosis, and gait disturbance are common. Limblength inequalities of as much as 10 cm have been reported in patients with unilateral dislocations. These patients develop flexion-adduction deformities of the hip, which may lead to valgus deformities of the knee. The valgus knee deformity is often associated with attenuation of the medial collateral ligament and degenerative joint disease of the lateral compartment, although some medial compartment disease has also been described (26, 58, 67, 118, 205) (Melvin P, Johnston R, Ponseti IV, personal communication). The same factors that are involved in the development of secondary degenerative disease in the false acetabulum and in the associated clinical disability in bilateral cases affect unilateral dislocations also.
Course of Dysplasia and Subluxation.
The natural history of dysplasia and subluxation in untreated patients is
important because of the likelihood that these findings can be extrapolated to residual dysplasia and subluxation after treatment (118, 204, 209, 210, 211, 212, 213 and 214).
important because of the likelihood that these findings can be extrapolated to residual dysplasia and subluxation after treatment (118, 204, 209, 210, 211, 212, 213 and 214).
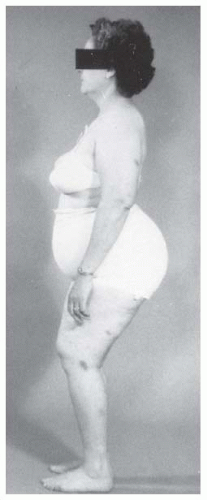 FIGURE 23-22. A 45-year-old woman with bilateral complete dislocations, hip flexion deformity, and marked hyperlordosis. The patient’s only reported symptoms concerned her back. |
After the neonatal period, the term dysplasia has an anatomic definition as well as a radiographic definition. Anatomic dysplasia refers to inadequate development of the acetabulum, the femoral head, or both (49). All subluxated hips (i.e., those in which there is some contact between the femoral head and the acetabulum) are by definition anatomically dysplastic. On film, the major difference between radiographic dysplasia and radiographic subluxation is determined by the integrity of the Shenton line. In radiographic subluxation, the Shenton line is disrupted and the femoral head is superiorly, laterally, or superolaterally displaced from the medial wall of the acetabulum. In radiographic dysplasia, the normal Shenton line relation is intact (58, 67, 215, 216) (Fig. 23-23). In the literature describing the natural history of DDH, these two radiographic and clinical entities are often not separated. Moreover, secondary degenerative changes may convert a radiographically dysplastic hip into a radiographically subluxated hip (206, 208, 212, 213, 216, 217 and 218) (Figs. 23-24 and 23-25).
Anatomic abnormalities are seen roentgenographically in subluxation and dysplasia, but the natural histories of these two radiographic entities are different. Residual radiographic subluxation after treatment of DDH invariably leads to degenerative joint disease and clinical disability (26, 66, 68, 118, 206, 211, 213, 216). The rate of deterioration is directly related to the severity of the subluxation and the age of the patient (213, 216).
There is considerable evidence that residual radiographic acetabular dysplasia leads to secondary degenerative joint disease, especially in women, although there are no predictive radiographic parameters (206, 212, 213, 216, 218, 219 and 220). The reasons for degenerative changes in radiographically dysplastic hips are probably mechanical in nature and related to increased contact stress over time. A certain threshold of “overpressure” (product of time and pressure, involving years of exposure to pressure above a 2-megapascal [MPa] level, leading to damage) (221, 222 and 223) may correlate with poor long-term outcome. Aspherical femoral heads (e.g., secondary to aseptic necrosis) tend to experience even more severe degrees of overpressure. It appears that radiographic degenerative joint disease correlates with the magnitude of the overpressure and the time of exposure (221). This overpressure may cause problems at a much younger age, with symptoms of “acetabular rim syndrome” (224) with associated labral tears, rim fractures and synovial cysts (225).
Physical signs of radiographic hip dysplasia may not be visible. Cases of radiographic hip dysplasia are diagnosed either only incidentally on the basis of radiographs taken for other reasons or after the patient develops symptoms (27, 28, 45, 77, 218). Stulberg and Harris found that 50% of their patients with radiographic dysplasia and degenerative joint disease had radiographic evidence of dysplasia in the other hip (218). Melvin et al., in their unpublished 30- to 50-year follow-up of DDH, demonstrated that 40% of the patients with DDH showed radiographic evidence of dysplasia in the opposite hip (Melvin P, Johnston R, Ponseti IV, personal communication) (225, 226 and 227). It has been estimated that 20% to 50% of degenerative joint disease of the hip is secondary to subluxation or residual radiographic acetabular dysplasia (45, 58, 216, 218, 219, 228, 229 and 230). Wiberg suggested that there was a direct correlation between the onset of radiographically determined degenerative joint disease and the amount of dysplasia as measured by the decrease in the CE angle (45) (Fig. 23-18).
Cooperman et al., in a radiographic study of degenerative joint disease and its relation to the severity of radiographic acetabular dysplasia, reviewed the 17 cases on which Wiberg based his conclusions (216). They concluded that 7 of 17 hips were actually subluxated. These subluxated hips were the most anatomically dysplastic; their CE angles averaged 2 degrees, and all seven had radiographically seen degenerative changes by the time the patients were 42 years of age. The other 10 hips in Wiberg’s series were radiographically dysplastic. They had intact Shenton lines and an average CE angle of 10 degrees. None of these patients developed radiographic degenerative joint disease before 39 years of age; however, degenerative changes became apparent radiographically by 57 years of age. In this review of Wiberg’s series, the decrease in CE angle was associated with an increase in anatomic acetabular dysplasia and an increased likelihood that the hip would be subluxated. Subluxation was the primary factor in the development of degenerative joint disease in this group. Subluxation predictably leads to degenerative joint disease and clinical disability over time.
Cooperman et al. described 32 hips (28 patients) with radiographic evidence of acetabular dysplasia (i.e., CE angle <20 degrees, but without subluxation and with the Shenton line intact), at an average follow-up of 22 years (216). All the patients eventually developed radiographic evidence of degenerative joint disease. However, there was no linear correlation between the CE angle and the rate of development of degenerative joint disease, as had been suggested previously by Wiberg. A decreased CE angle was associated solely with increasing radiographic evidence of acetabular dysplasia and not with subluxation, because patients with subluxation had been excluded in this series. Cooperman et al. demonstrated that radiographic evidence of acetabular dysplasia leads to radiographically detectable degenerative joint disease, but the process may take decades. This study also demonstrated that the conventional radiographic parameters used for describing dysplasia (e.g., CE angle, acetabular index of Sharp, percent coverage, depth, inclination) could not predict the rate at which a radiographically confirmed dysplastic hip joint would develop radiographic evidence of degenerative joint disease.
Stulberg and Harris demonstrated that there is no radiographic picture of degenerative joint disease that is uniquely associated with preexisting acetabular dysplasia (218). In 80% of patients with dysplasia, the CE angle is usually <20 degrees, but acetabular shallowness, as measured by acetabular depth, affects all of these patients. The investigators also demonstrated that the CE angle, the criterion most commonly used to quantitate dysplasia, could be affected by many factors, including positioning for the radiographs and the changes accompanying the normal development of degenerative joint disease. The secondary degenerative changes in a dysplastic acetabulum may give the hip a normal-appearing CE angle. In their series of 130 patients with primary or idiopathic degenerative joint disease, Stulberg and Harris were able to demonstrate that 48% showed evidence of primary acetabular dysplasia and that acetabular dysplasia frequently occurred in women with degenerative joint disease.
Additional evidence for the association between radiographic evidence of acetabular dysplasia and degenerative joint disease comes from the southern Chinese population. In an epidemiologic study from Hong Kong, where the incidence of childhood hip disease is low, the incidence of adult osteoarthritis (nontraumatic) was also shown to be low (54, 55).
Wedge and Wasylenko reported three peak periods of pain in subluxation, depending on the severity of the subluxation (58, 67). Patients with the most severe subluxation usually had the onset of symptoms during the second decade of life. Those with moderate subluxation presented during their third and fourth decades, and those with minimal subluxation usually experienced the onset of symptoms around the fifth decade.
Patients who present soon after the onset of symptoms rarely have the classic signs of degenerative joint disease such as decreased joint space, cyst formation, double acetabular floor, and inferomedial femoral head osteophytes. The only radiographic feature present at the onset of symptoms may be increased sclerosis in the weight-bearing area. This increased sclerosis is secondary to increasing osteoblastic stimulation in response to the decreased weight-bearing surface area; the increase of the normal per unit load strains the bone. The mechanism of pain in these instances is open to speculation.
In cases of subluxation, the mean age at onset of symptoms is 36.6 years in women and 54 years in men. Severe degenerative changes become evident radiographically approximately 10 years later by 46.4 years of age in women and 69.6 years of age in men.
Patients with subluxated hips usually experience onset of symptoms at a younger age than patients with complete dislocations do. After pain and radiographically evident degenerative disease start, the disease progresses rapidly. Harris reported that symptoms of degenerative joint disease associated with radiographic evidence of acetabular dysplasia occurred early in life and that almost 50% of the patients in his series with acetabular dysplasia had their first reconstructive procedure before 60 years of age, with fewer than 5% having their first reconstruction after 60 years of age (219).
At maturity, the ideal radiograph should have a well-developed teardrop, a normal femoral neck-shaft angle, an intact Shenton line, a downsloping sourcil, and a well-developed gothic arch (Fig. 23-26). Any deviation from this radiographic appearance may lead to degenerative joint disease over the long term (58, 118, 188, 212, 213, 216, 217 and 218)
One of the most controversial areas in the discussion of hip dysplasia either primary or as a residual of treatment is the effect of acetabular retroversion and/or femoral acetabular impingement (FAI) on the natural history or long-term outcome of patients.
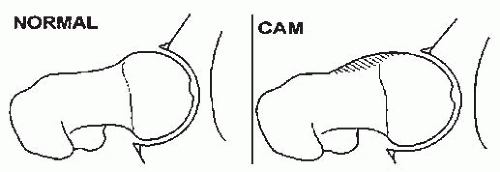
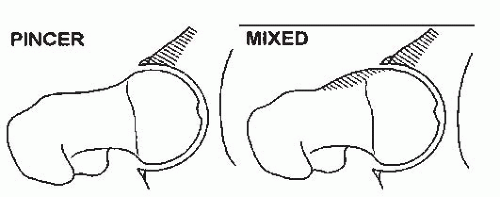
There is increasing evidence that FAI may lead to acetabular cartilage delamination and degenerative joint disease (231, 232, 233, 234, 235, 236, 237 and 238) FAI is more commonly associated with a retroverted acetabulum which can be seen in cases of hip dysplasia (x-ray cross-over sign). Two types of impingement have been described: Cam and Pincer (Figs. 23-27 and 23-28) (239). While cam impingement may be determined by plane radiographs, particularly the lateral view with the hip in internal rotation, contrast-enhanced MRI is often used to gain a broader, more in-depth view of the anatomic abnormalities thought to be associated with FAI. Cam impingement results from deformities of the proximal part of the femur that may occur in patients with DDH particularly those treated late; while Pincer impingement generally is associated with acetabular overcoverage, both types may be present in the same patient (240, 241). While these anatomic findings are thought to be associated with degenerative joint disease, what is uncertain is the presence of these radiographic findings in the general population. Further studies will be necessary to clarify the incidence of retroversion and radiographic abnormalities of the proximal femur in the general population.
TREATMENT OF HIP DISLOCATION
Newborns and Infants Younger Than 6 Months of Age.
On the basis of the understanding of normal growth and development of the hip, the fundamental treatment goals in DDH are the same, regardless of the age of the patient. The first goal is to obtain reduction and maintain that reduction to provide an optimal environment for the development of the femoral head and acetabulum (117). As has been demonstrated by many follow-up studies of treated DDH, the acetabulum has the potential for development for many years after reduction as long as the reduction is maintained (35, 37, 38). The femoral head and femoral anteversion can remodel if the reduction is maintained (213, 242). Further intervention is necessary only to alter an otherwise adverse natural history, as in the treatment of residual dysplasia and the prevention or treatment of subluxation. The later the diagnosis of DDH is made, the more difficult it is to achieve these goals, the less potential there is for acetabular and proximal femoral remodeling, and the more complex are the required treatments. With increasing age and complexity of treatment, the risk of complications is greater, and the patient is more likely to develop degenerative joint disease.
The diagnosis of DDH should ideally be made in the newborn nursery (243). If the diagnosis is made in the nursery, treatment should be initiated immediately (244). Triple diapers or abduction diapers have no place in the treatment of DDH in the newborn. They give the family a false sense of security and are generally ineffective. Any success with the use of triple diapers or abduction diapers could be attributed to the natural resolution of the disorder.
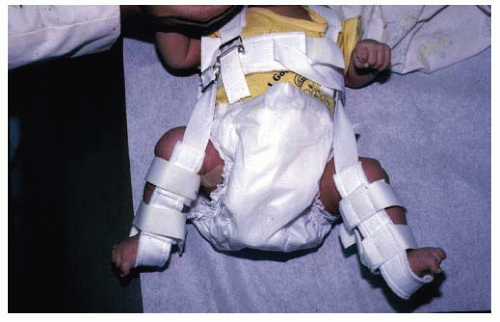
The most commonly used device for the treatment of DDH in the newborn is the Pavlik harness (Fig. 23-29). Although other devices are available (e.g., von Rosen splint, Frejka pillow), the Pavlik harness remains the most commonly used device worldwide (36, 83, 245, 246, 247, 248, 249, 250, 251, 252, 253, 254, 255, 256 and 257). When appropriately applied, the Pavlik harness prevents the hip extension and adduction that can lead to redislocation, but it allows further flexion and abduction, which lead to reduction and stabilization. By maintaining the Ortolani-positive hip in a Pavlik harness on a full-time basis for 6 weeks, hip instability resolves in 95% of cases (257).
The Pavlik harness may be used effectively until 6 months of age for any child with residual dysplasia, subluxation, or complete dislocation. After 6 months of age, the failure rate for the Pavlik harness is >50%, because it is difficult to maintain the increasingly active and crawling child in the harness.
Mubarak et al. and others described the disadvantages associated with the use of the Pavlik harness for the treatment of DDH (34, 258). They pointed out that failures of treatment most often result from problems related to the physician, the device, or the patient.
The physician-related errors fall into two categories: inappropriate application and persistence of inadequate treatment. The Pavlik harness is contraindicated in patients with significant muscle imbalance, such as those with myelodysplasia or cerebral palsy. It is also contraindicated in patients who have significant stiffness of the joints, such as children with arthrogryposis. The harness will fail if it is applied in a child with excessive ligamentous laxity, as seen in Ehlers-Danlos syndrome (34).
The persistence of inadequate treatment is the result of many factors. If treatment with the harness is to be successful, physicians should be well-versed in its appropriate application and the adjustments that are necessary throughout the course of treatment. It is important that the physician recognize when a treatment failure has occurred, so as not to prolong treatment with the harness and cause secondary pathologic changes, called Pavlik harness disease (259). Persistence of treatment may damage the femoral head, injure the acetabular cartilage, and impair future bone growth. An inappropriately applied harness is a failure of the physician, not a failure of the orthotic (258, 260, 261).
Another major Pavlik harness problem is related to the specific orthotic device. Not all Pavlik harnesses are the same; the strap attachment sites vary. However, since the article by Mubarak et al. in 1981, most harnesses on the market meet the requisite standards that those authors outlined (34, 258).
Some problems are related to the patient. Certain family, social, and educational situations make compliance impossible. In these situations, the Pavlik harness would be inappropriate, and closed reduction and casting may be the more judicious approach. The family must be educated about the importance of the harness, its care and maintenance, how the child should be bathed while wearing the harness, and the consequences of failure. Family noncompliance can lead to failure, and the use of a visiting nurse may be helpful in these situations.
The method of application of the harness (Fig. 23-29) should be demonstrated to the family members. The chest halter strap should be positioned at the nipple line, and the shoulder straps are to be set to hold the cross strap at this level. The leg and foot stirrups must have their anterior and posterior straps oriented anteriorly and posteriorly to the child’s knees. Hip flexion should be set at 100 to 110 degrees. These straps should be in the anterior axillary line. The posterior abduction strap should be at the level of the child’s scapula and adjusted to allow comfortable abduction within the safe zone (262), which is defined as the arc of abduction and adduction, that is, between redislocation and comfortable, unforced abduction. The posterior strap acts as a checkrein to prevent the hip from adducting to the point of redislocation. Ultrasonography is a useful means of documenting relocation of the Ortolani-positive hip.
There is great variability in treatment regimens with the Pavlik harness. If the Pavlik harness is used for stabilizing an unstable hip (i.e., an Ortolani- or Barlow-positive hip), the harness is used full time for 6 to 12 weeks after clinical stability is achieved. The author’s preference is to use the harness full time for 6 weeks beyond the point when stability is reached. Most hips stabilize in days to weeks. The harness is checked at 7- to 10-day intervals to assess hip stability and to adjust the flexion and abduction straps to allow for growth of the infant. In the author’s opinion, clinical examination is usually sufficient to check on the progress at each visit; but if uncertainty is present, ultrasonography may be used; radiographs are unnecessary.
In a child younger than 6 months who has a complete dislocation, the Pavlik harness may be used in a trial of
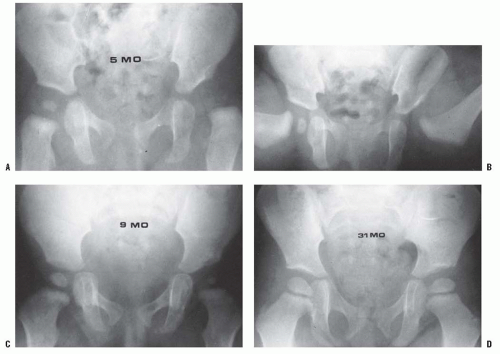
ultrasound-monitored reduction. In this case, the harness must be applied with enough hyperflexion and abduction to point the femoral head toward the triradiate cartilage. This situation is the ideal indication for the use of ultrasonography to follow the reduction. When the harness is used in this situation, the infant should be checked at 7 to 10 days to determine whether the reduction is being accomplished. Clinical examination alone may be adequate, but initial radiographs or ultrasound should be obtained in order to document adequate flexion and redirection of the femoral neck toward the triradiate cartilage in the harness. After clinical stability is achieved, radiography is not indicated until approximately 3 months of age to assess acetabular development (Fig. 23-30). Ultrasonography is an excellent means of documenting progress toward and completion of successful reduction (263).
ultrasound-monitored reduction. In this case, the harness must be applied with enough hyperflexion and abduction to point the femoral head toward the triradiate cartilage. This situation is the ideal indication for the use of ultrasonography to follow the reduction. When the harness is used in this situation, the infant should be checked at 7 to 10 days to determine whether the reduction is being accomplished. Clinical examination alone may be adequate, but initial radiographs or ultrasound should be obtained in order to document adequate flexion and redirection of the femoral neck toward the triradiate cartilage in the harness. After clinical stability is achieved, radiography is not indicated until approximately 3 months of age to assess acetabular development (Fig. 23-30). Ultrasonography is an excellent means of documenting progress toward and completion of successful reduction (263).
Although the Pavlik harness has provided a 95% overall success rate for the treatment of the Ortolani-positive hip, the success rate for using the harness to guide the reduction of a subluxated or dislocated hip in a child younger than 6 months of age is 85% (34, 249, 257, 264, 265).
The use of the Pavlik harness can be associated with complications; most of these complications are iatrogenic and can be avoided. Inferior dislocations may occur with prolonged excessive hip flexion (266, 267 and 268). Hyperflexion may also induce femoral nerve compression neuropathy; this condition generally resolves after the harness is removed. It is important during each examination to make certain that the patient has active quadriceps function. Brachial plexus palsy may occur from compression by the shoulder straps, and knee subluxations may occur from improperly positioned straps.
Skin breakdown may occur in the groin creases and in the popliteal fossa if great care is not taken in keeping these areas clean and dry. Instruction with regard to bathing and skin care is essential.
The most disastrous consequence of Pavlik harness treatment is damage to the cartilaginous femoral head and the proximal femoral physeal plate (269, 270). This is usually secondary to forced abduction in the harness or to persistent use of the harness, despite the failure of reduction, in a complete dislocation.
Children 6 Months to 2 Years of Age.
It is difficult to maintain a child older than 6 months of age in a Pavlik harness because of the child’s activity levels. In this age group, subluxated or dislocated hips should be treated by closed or open means as necessary, because success rates using the Pavlik harness are <50%.
In the late-diagnosed patient or the patient who fails treatment with the Pavlik harness, the obstacles to reduction are different, treatment has greater risks, and the results are far less predictable. The principal goals in the treatment of the late-diagnosed patient are similar to those for the newborn. The goal is to obtain reduction, to maintain that reduction so as to provide an adequate environment for femoral head and acetabular development, and to avoid proximal femoral growth disturbance (Fig. 23-31).
Traction.
For patients older than 6 months of age at diagnosis and those who have failed a trial of Pavlik harness reduction, closed reduction is indicated. In some centers, treatment by closed reduction and spica cast immobilization is preceded by a period of skin or skeletal traction (271, 272, 273, 274, 275, 276, 277, 278, 279, 280, 281 and 282) (Fig. 23-32). Traction theoretically stretches contracted muscles, allows reduction without excessive force, decreases the need for open reduction, and reduces the incidence of proximal femoral growth disturbance resulting from avascular necrosis (AVN). The use of prereduction traction, and its effectiveness at accomplishing what it is touted to do, are controversial topics (283, 284 and 285). In 1991, Fish et al. sought the opinions of the members of the Pediatric Orthopaedic Society of North America on this topic (286). Most pediatric orthopaedic surgeons thought that traction did reduce the incidence of necrosis in the treatment of DDH. Only 5% of responders did not use traction in their practice. In the author’s opinion, this practice pattern has changed considerably over the last 20 years, with far fewer pediatric orthopaedic surgeons employing prereduction traction.
The purpose of traction is to allow gradual relaxation of secondarily contracted muscles, such as the iliopsoas and adductor longus; theoretically, this allows reduction without creating excessive joint forces, thereby decreasing the incidence of AVN and reducing the need for open reduction. These ideas are lacking the support of scientifically valid studies (284).
Gage and Winter studied a group of patients in order to quantify prereduction hip positions and concluded that there was a direct correlation between inadequate traction and the incidence of growth disturbance (274). Weiner et al. found that in patients younger than 1 year traction for longer than 21 days substantially reduced the rate of growth disturbance (279). Buchanan et al. recommended a minimum of 2 weeks of traction until achievement of a 2+ traction station using the Gage and Winter scale (271). Skeletal traction was gradually increased over several weeks, and an average of 39% of body weight was usually required for achieving this position. In contrast, Cooperman et al. studied 30 DDH hips with aseptic necrosis and 30 hips without necrosis and found, at an average 39-year follow-up, that the degree of initial displacement that had to be overcome in order to obtain reduction was comparable in both groups and that it was not a factor in the development of proximal femoral growth disturbance (287). Some of the worst outcomes were seen in patients with minimal superior dislocation. Schoenecker and Strecker demonstrated that the results of traction were not as good as the results of femoral shortening in older patients with DDH (288). Gibson and Benson (214) thought that although preliminary traction protects against growth disturbance, there was no relation between the original degree of displacement of the proximal femur and the final outcome (289). A study by Kutlu et al. (290), looking at traction as a single variable in a series of patients treated by closed reduction, showed that traction did not affect the rate of necrosis.
With respect to traction facilitating reduction, the assessment of the adequacy of closed reduction and the need for open reduction varies and is subjective. Several articles on open and closed reduction without the use of preliminary traction report incidences of proximal femoral damage comparable to those found in series in which prereduction traction was used (44, 291, 292 and 293). These researchers think that the main obstacles to reduction are intraarticular and therefore would not be affected by the use of traction. Controversy also exists about the amount of weight applied, the direction of application of the force, and the duration of applied traction. There are no clinical or experimental studies of the direct effects of traction, and there are no well-controlled studies that analyze the effect of traction as a single variable (284).
Surgeons who choose to use prereduction traction generally consider that 1 to 2 weeks of skin or skeletal traction are sufficient. However, a report on the successful use of traction to attain reduction in patients more than 6 months of age reported a mean time in traction of 8 weeks (294). Skin traction is the most commonly used method, although some physicians recommend skeletal traction (260). Skin tapes should be applied above the knee to distribute the traction over a large area (Fig. 23-32). Elastoplast tape is applied loosely over tincture of benzoin from the ankle to the upper thigh. It is important not to stretch the Elastoplast tape at all; it should merely lie on the skin in a circumferential manner, with each edge directly opposing the preceding edge. Buck traction tapes are then applied from above the ankle to the thigh and to the foot plate; weights may be added to both legs, so that the buttocks “lightly” touch the bed. The author has used this method without adverse consequences. The direction of application of the traction forces (e.g., overhead, longitudinal, divaricated) and the duration of traction (days to months) vary worldwide.
The complications of traction include skin loss and ischemia of the lower extremities; these are attributable to inappropriate application. Neurocirculatory checks must be performed frequently, and traction must be applied in a carefully supervised manner.
In appropriate circumstances, traction may be used at home (295, 296 and 297). This markedly decreases the costs associated with hospitalization. Patients are usually hospitalized for 24 hours to allow their parents to become familiar with the traction apparatus, to learn how to monitor neurocirculatory status, and to become totally familiar with the potential risks and danger signs. The patient and family must be cooperative; a visiting nurse is often helpful in instituting this program.
Closed Reduction.
Closed reductions are performed in the operating room. Gentle reduction must be done under general anesthesia. The hip is gently manipulated into the acetabulum by flexion, traction, and abduction. An open or percutaneous adductor tenotomy is usually necessary in these cases because of secondary adduction contracture, and for increasing the “safe zone” (arc of adduction-abduction in which the hip remains located), thereby lessening the incidence of proximal femoral growth disturbance. In the author’s hands, if adductor tenotomy is done open, then a psoas tenotomy is also completed.
The adequacy of closed reduction is somewhat subjective. In the author’s anatomic reduction is the only acceptable reduction (Fig. 23-33). Because large portions of the femoral head and acetabulum are cartilaginous, arthrography is a useful tool in assessing the obstacles to and the adequacy of reduction (296, 298, 299, 300, 301 and 302). Dynamic arthrography using fluoroscopy helps to achieve both of these goals. Intraoperative ultrasonography may also be used. The use of the femoral head as a “dilating sound” to overcome the intraarticular obstacles to reduction may cause damage to the femoral head and make open reduction more difficult (259, 303, 304).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree