Chapter 25 Débridement and Microfracture for Full-Thickness Articular Cartilage Defects
Background
Full-thickness articular cartilage defects in the knee are common, and the lesions may present in a variety of clinical settings and at different ages.11 A single event, the shearing forces of the femur on the tibia, may result in trauma to the articular cartilage, causing the cartilage to fracture, lacerate, and separate from the underlying subchondral bone or to separate with a piece of the subchondral bone. Alternatively, chronic repetitive loading in excess of normal physiologic levels may result in the fatigue and failure of the articular surface, especially in the presence of meniscus deficiency or axial malalignment. The single events are usually found in younger groups, whereas chronic degenerative lesions are seen in the middle-aged and older groups. It has been shown that repetitive impacts can cause cartilage swelling, an increase in collagen fiber diameter, and an alteration in the relationship between collagen and proteoglycans. Thus, acute events may not result in full-thickness cartilage loss but rather start a degenerative cascade that can lead to chronic full-thickness loss. The degenerative cascade typically includes early softening and fibrillation (grade I), fissures and cracks in the surface of the cartilage (grade II), severe fissures and cracks with a “crab meat” appearance (grade III) and, finally, exposure of the subchondral bone (grade IV).
Articular cartilage defects that extend full thickness to subchondral bone rarely heal without intervention.13–1821 Some patients may not develop clinically significant problems from acute full-thickness chondral defects, but most eventually suffer from degenerative changes that can be debilitating. Techniques used to treat chondral defects include marrow stimulation, osteochondral autografts, osteochondral allografts, and autologous cell transplantation. The senior author (JRS) developed the microfracture technique to enhance chondral resurfacing by providing a suitable environment for new tissue formation and taking advantage of the body’s own healing potential. The senior author’s clinical experience now includes more than 3500 patients in whom microfracture has been done.
We have found that arthroscopic débridement accompanied by microfracture of subchondral bone is a reliable and repeatable procedure to stimulate biologic repair of cartilage defects of the knee in patients in whom nonoperative treatment has failed or in whom acute lesions were encountered during arthroscopy. Thus, more invasive procedures, such as osteotomy, cartilage grafting, or unicompartmental arthroplasty, might be avoided or at least delayed for several years. Specifically, the goals of this procedure are to alleviate the pain and attendant disabilities that result from the chondral lesions and also to prevent late degenerative changes in the joint by restoring the joint surface.13,15
Indications for Microfracture
Microfracture was developed initially for patients with post-traumatic articular cartilage lesions of the knee that had progressed to full-thickness chondral defects. The microfracture technique still is most commonly indicated for full-thickness loss of articular cartilage in either a weight-bearing area between the femur and tibia or in an area of contact between the patella and trochlear groove.13–18 Unstable cartilage that overlies the subchondral bone is also an indication for microfracture. If a partial-thickness lesion is probed and the cartilage simply scrapes off down to bone, we consider this to be a full-thickness lesion. Degenerative joint disease in a knee that has proper axial alignment is another common indication for microfracture. These lesions all involve loss of articular cartilage at the bone-cartilage interface.
Patients with acute chondral injuries are treated as soon as practical after the diagnosis is made, especially if the knee is being treated concurrently for meniscus or anterior cruciate ligament pathology. Patients with chronic or degenerative chondral lesions often are treated nonoperatively (conservatively) for at least 12 weeks after a suspected chondral lesion is diagnosed clinically. This treatment regimen includes activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, joint injections, and perhaps dietary supplements that may have cartilage-stimulating properties. If nonoperative treatment is not successful, surgical treatment is considered.13–18
No limitations are placed on how large an acute lesion can be and still be considered suitable for microfracture.15,18 We have observed that even very large acute lesions respond well to microfracture.13 We have noted empirically that traumatic lesions, acute or chronic, smaller than 400 mm2 tend to respond better to microfracture than those lesions larger than 400 mm2, but we have not observed this difference to be statistically significant.12 Treatment of chronic degenerative lesions is not specifically limited by size, but more emphasis is placed on proper axial alignment and the presence of global degenerative changes throughout the knee.
General considerations for the use of the microfracture procedure include patient age, acceptable biomechanical alignment of the knee, patient’s activity level, and patient’s expectations.12–17 If all these criteria define a patient who could benefit from chondral resurfacing, then microfracture is considered.
Contraindications for Microfracture
Specific contraindications for microfracture include axial malalignment (see later), patients unwilling or unable to follow the required strict and rigorous rehabilitation protocol, partial-thickness defects, and inability to use the opposite leg for weight bearing during the minimal or non–weight-bearing time.15,16 Other specific contraindications include any systemic immune-mediated disease, disease-induced arthritis, or cartilage disease. A relative contraindication is for patients older than 65 years because we have observed that some patients older than 65 years experience difficulty with crutch walking and the required rigorous rehabilitation. Other contraindications to microfracture include global degenerative osteoarthrosis or the cartilage surrounding the lesion is too thin to establish a perpendicular rim to hold the marrow clot.12 In these advanced degenerative cases, axial malalignment often is also a confounding factor.
Two methods for radiographic measurement of the biomechanical alignment of the weight-bearing axis of the knee are used in our facility: (1) the angle made between the femur and tibia on anteroposterior (AP) views obtained with the patient standing; and (2) the weight-bearing mechanical axis drawn from the center of the femoral head to the center of the tibiotarsal joint on long standing (~51 inch [130 cm]) radiographs. If the angle drawn between the tibia and femur is greater than 5 degrees of varus or valgus compared with the normal knee, this amount of axial malalignment would be a relative contraindication for microfracture. Preferably, the mechanical axis weight-bearing line should be in the central 25% of the tibial plateau of the medial or lateral compartment. If the mechanical axis weight-bearing line falls outside the centralmost 25% of the plateaus, medial or lateral, this weight-bearing shift also would be a relative contraindication if left uncorrected. In such cases, a realignment procedure should be included as part of the overall treatment regimen.12,13
Surgical Technique
Three portals are routinely made about the knee for use of the inflow cannula, the arthroscope, and the working instruments. We typically do not use a tourniquet during the microfracture procedure; rather, we vary the arthroscopic fluid pump pressure to control bleeding. An initial, thorough, diagnostic examination of the knee should be done. We carefully inspect all geographic areas of the knee, including the suprapatellar pouch, the medial and lateral gutters, the patellofemoral joint, the intercondylar notch and its contents, the anterior interval between the patellar tendon and tibia, and the medial and lateral compartments, including the posterior horns of both menisci. We do all other intra-articular procedures before doing the microfracture. This technique helps prevent loss of visualization when the fat droplets and blood enter the knee from the microfracture holes, and the marrow clot is less likely to be dislodged. Importantly, we pay particular attention to soft tissues such as plicae and the lateral retinaculum, which potentially could produce increased compression between cartilage surfaces.13–18
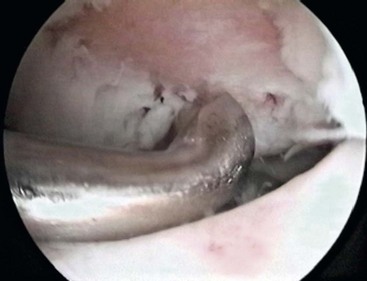
After carefully assessing the full thickness articular cartilage lesion, we débride the exposed bone of all remaining unstable cartilage. We use a hand-held curved curette and a full-radius resector to débride the cartilage. It is critical to débride all loose or marginally attached cartilage from the surrounding rim of the lesion. The calcified cartilage layer that remains as a cap to many lesions must be removed, preferably by using a curette. Thorough and complete removal of the calcified cartilage layer is extremely important based on animal studies we have completed.2,3 Care should be taken to maintain the integrity of the subchondral plate by not débriding too deeply. This prepared lesion, with a stable perpendicular edge of healthy, well-attached, viable cartilage surrounding the defect, provides a pool that helps hold the marrow clot (or superclot, as we have termed it) as it forms (Fig. 25-1).15
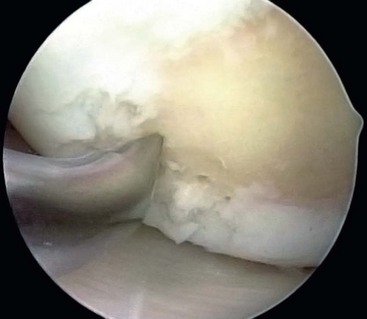
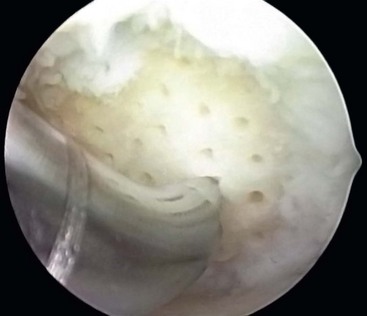
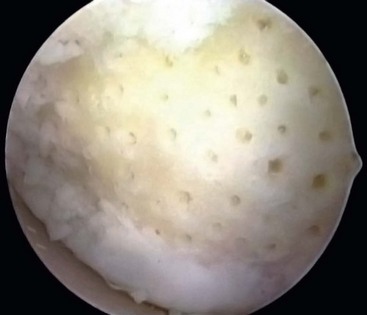
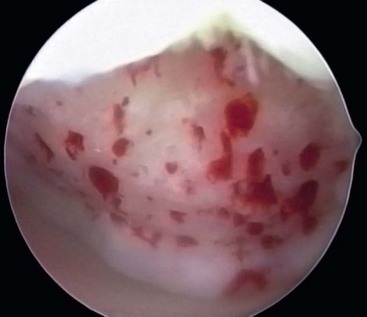
After preparation of the lesion, we use an arthroscopic awl to make multiple holes, or microfractures, in the exposed subchondral bone plate. We use an awl with an angle that permits the tip to be perpendicular to the bone as it is advanced, typically 30 or 45 degrees. There also is a 90-degree awl that typically is used only on the patella or other soft bone. The 90-degree awl should only be advanced manually, not with a mallet. The holes are made as close together as possible, but not so close that one breaks into another, thus damaging the subchondral plate between them. This technique usually results in microfracture holes that are approximately 3 to 4 mm apart. When fat droplets can be seen coming from the marrow cavity, the appropriate depth (≈2 to 4 mm) has been reached. The arthroscopic awls likely produce essentially no thermal necrosis of the bone compared with hand-driven or motorized drills. We make microfracture holes around the periphery of the defect first, immediately adjacent to the healthy stable cartilage rim (Fig. 25-2). Then we complete the process by making the microfracture holes toward the center of the defect (Fig. 25-3). We assess the treated lesion at the conclusion of the microfracture to ensure that a sufficient number of holes have been made before we reduce the arthroscopic irrigation fluid flow (Fig. 25-4). After the arthroscopic irrigation fluid pump pressure is reduced, we are able to observe under direct visualization the release of marrow fat droplets and blood from the microfracture holes into the subchondral bone. We judge the quantity of marrow contents flowing into the joint to be adequate when we observe marrow emanating from all microfracture holes (Fig. 25-5). We then remove all instruments from the knee and evacuate the joint of fluid.15,16 Intra-articular drains should not be used because the goal is for the surgically induced marrow clot, rich in marrow elements, to form and stabilize while covering the lesion.2,3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree