Fig. 23.1
Basic concept of tissue engineering. The use of a combination of cells, scaffolds, and growth factors improves or restores biological function
Anatomy of the Cartilage, Subchondral Bone, and Their Interface
The osteochondral complex consists of both the articular cartilage and underlying subchondral bone. Biochemically, cartilage tissue is largely comprised of water, chondrocytes, type II collagen, and proteoglycan [50, 81, 122]. The cartilage can be differentiated into four distinct zones: the superficial, middle, deep, and calcified cartilage zones (Fig. 23.2) [65]. Each zone is defined by a particular composition and organization of cells and extracellular matrix (ECM) molecules. The differential proportions in ECM composition influence the mechanical properties of each zone of the cartilage. For example, the superficial zone is strong in tension along the alignment of its collagen fibrils, thereby assisting in the resistance of shear forces at the surface. By comparison, the deep zone has more compressive strain.
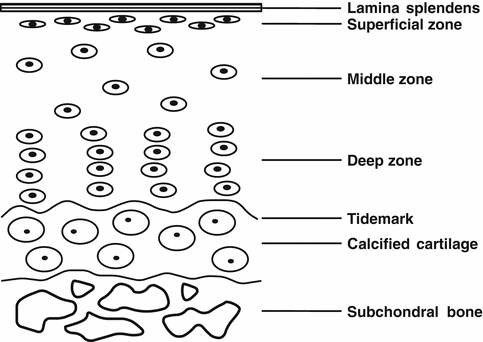

Fig. 23.2
Schematic drawing of the different zones of articular cartilage and subchondral bone
Subchondral bone is a complex tissue consisting of water, collagen type I, and hydroxyapatite, with the two latter components providing the tissue’s stiffness and compressive strength [9, 81, 122]. The compressive modulus of subchondral bone is higher than that of the cartilage. The different morphological compositions and mechanical properties of subchondral bone and cartilage indicate the complexity of the tissue interface.
The osteochondral interface is described by the interaction of calcified cartilage and the underlying subchondral bone [16]. Structurally, collagen fibers extend from the deep zone to calcified cartilage through a wavy tidemark, which enables the dispersal of force through the vertical orientation of collagen fibrils [83]. However, despite the fact that calcified cartilage is mineralized tissue, its mechanical strength is lower than that of the subchondral bone [72]. Calcified cartilage is interdigitated with subchondral bone, but fibers do not extend across the zone into the bone [23, 83]. The wavy tidemark and vertically oriented fibers at the tidemark, as well as interdigitations present at the interface, may allow for reducing stress concentrations, as well as better integration with the underlying subchondral bone [81, 83].
Characteristic of Osteoarthritic Joint
An osteoarthritic joint is characterized by degenerative changes, such as articular cartilage loss, subchondral bone thickening, and osteophyte formation [12, 39, 64, 77, 96]. The primary morphologic changes include thinning, fissuring, and fragmentation of articular cartilage. With the progression of the disease comes a continuous loss of articular cartilage, accompanied with the decrease of collagen type II and aggrecan [31, 76], leading to exposure of subchondral bone. Secondary changes are frequently seen in the underlying bone, such as sclerosis, cystic change, and new bone formation (Fig. 23.3a, b). These changes are considered to be triggered by a multitude of factors, including aging, trauma, obesity, mechanical overload, congenital disorder, and infection, which do not heal spontaneously once damaged.
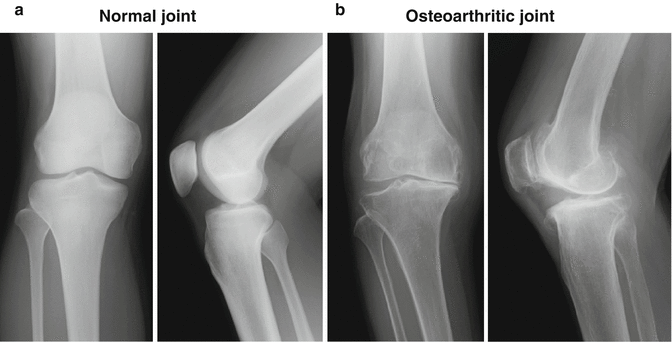

Fig. 23.3
Radiography of (a) normal healthy knee joint and (b) osteoarthritic knee joint. In osteoarthritis, the loss of the cartilage (joint space narrowing) and subchondral bone change such as sclerosis, cystic change, and new bone formation (osteophyte) are frequently seen
Strategy for Osteochondral Repair
For an ideal repair of osteochondral lesions, it is important to regenerate subchondral bone and to facilitate zonal restoration of the cartilage and subchondral bone, layer by layer, mimicking the natural articular structure [37, 46, 53, 74, 85, 86, 102]. As a strategy to regenerate these structures in a layer-by-layer fashion, biphasic or triphasic constructs have been developed due to both mechanical and biological reasons, including the acquisition of initial mechanical strength, mimicking a natural articulate structure, a uniform tidemark at the osteochondral junction, and an integration of the biphasic implant with host tissue to sustain biological function (Fig. 23.4a–d) [2, 4, 18, 36, 43, 47, 69, 82, 84, 107, 110]. For satisfying the biological requirements, an osteochondral implant should ideally have a rigid osseous layer to support the overlying cartilage and integrate with the native bone and a chondral layer to allow the seeding and proliferation of chondrocytes or mesenchymal stem cells (MSCs) and subsequent deposition of cartilaginous ECM. Also, for the successful osteochondral repair, the integration between the implant and host tissue should be one of crucial factors. Our previous study showed that the tissue integration to native surrounding osteochondral tissue after the implantation of biphasic construct could influence the quality and maturation of repair tissue [109]. In case of the failure of integration with the adjacent host cartilage, the repair tissue might evolve into a pathological condition such as OA due to mechanically unstable condition, which potentially raises concerns regarding the long-term durability of the repair tissue.
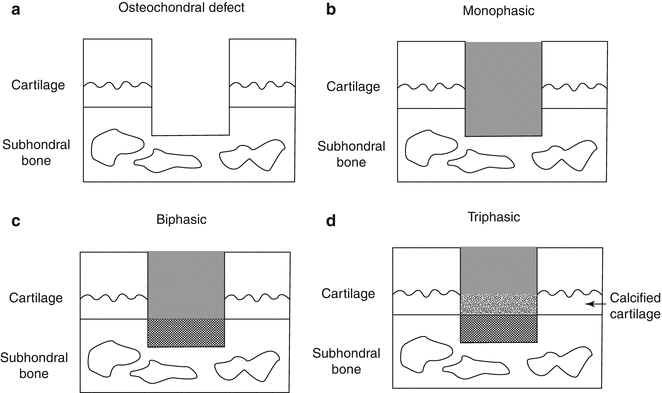

Fig. 23.4
Schematic drawing of (a) osteochondral defect and (b) implantation of monophasic scaffold, (c) biphasic scaffold, and (d) triphasic scaffold
Design and Fabrication of Biomaterial Scaffold
In general, osteochondral tissue engineering strategies can be categorized into monophasic (Fig. 23.4b) and biphasic (Fig. 23.4c) depending on the biological and biomechanical characteristics of the scaffold. As mentioned above, a successful tissue engineering approach for osteochondral repair involves the design of a biphasic scaffold with the potential to regenerate both the cartilage and subchondral bone. The fabrication of the majority of scaffolds is performed through independent processes, whereby different scaffolds for the two sides are created and then combined, or via a simultaneous process through which a single scaffold is created and cultured simultaneously for both sides [67, 81]. A biphasic construct developed independently allows the cultivation of both chondrogenic and osteogenic cells in separate media and environmental conditions. However, these constructs must be hybridized into a single composite graft by connecting the two layers together. The potential disadvantage of this approach might be the difficulty in achieving a secure biological and mechanical integration between the two layers [67]. On the other hand, when the two layers are hybridized prior to culture, a complicated system will be required to promote osteo- and chondral differentiation separately in each layer. Due to the difficulty of two different cell cultures simultaneously, such pre-developed biphasic constructs are mainly used as a cell-free scaffold [53].
Some research groups have raised the importance of an intermediate layer between the cartilage and subchondral bone layers to represent the tidemark or calcified cartilage; triphasic scaffolds were therefore developed (Fig. 23.4d) [53, 69]. However, the intermediate layer has unique osteochondral characteristics owing to the infiltration of the blood vessels, and thus, it may be difficult to mimic the unique structure with currently available biomaterial technologies. In fact, the superiority of triphasic scaffolds over biphasic for osteochondral repair has not yet been demonstrated and requires further investigation.
Most scaffolds have the pore structures inside, which would affect the regulation of cell invasion, vascularization, and tissue maturation [105]. The pore size and porosity should be controlled suitable for tissue engineering in the fabrication process of porous scaffolds. Regarding the effects of pore size on osteogenesis, the scaffold structure composed of porosity higher than 50 % and pores larger than 300 μm is recommended to achieve direct osteogenesis with enhanced vascularization [48]. On the contrary, the scaffold with smaller pores have been suggested for favorable chondrogenesis on 90–120 μm pores, in which MSCs proliferate and promote chondrogenesis in the scaffold [51].
Recent advances in computer-aided tissue engineering including three-dimensional (3D) printing technique enable the fabrication of multifunctional scaffolds that meet the microstructural, mechanical, and nutritional requirements based on optimized models [59, 63]. Moreover, these techniques will be expected to generate the custom-shaped engineered grafts from clinical imaging data with the use of CT or MRI, which fit the specific defect [79]. Therefore, the bioprinting technology should be a powerful tool for building tissues at cellular and organ levels.
Choice of Cells and Growth Factors
The most direct cell source may be the biopsy specimens taken from the patients, from which mature osteoblasts and chondrocytes may be obtained. However, as the number of cells obtained is usually limited, it is typically not enough to allow seeding onto the scaffolds. Also, expansion of primary cells may result in a loss of differentiation capacity; for example, the expansion of articular chondrocytes can lead to dedifferentiation into fibroblast [13, 104, 115]. To overcome such potential problems with respect to dedifferentiation, three-dimensional (3D) culture can be used to retain the cellular phenotype and avoid dedifferentiation [116]. The most common method is the use of various scaffolds to produce a 3D culture condition [68, 128] and may be combined with the supplementation of growth factors [22], the use of bioreactor [35], the mechanical stimulation of the cells [33, 49], and the use of low oxygen tension [57] during cultivation. Also, even if chondrocytes lose their differentiated phenotype, dedifferentiated chondrocytes can regain their differentiated phenotype through the redifferentiation process of cultivation in a 3D scaffold combined with growth factors [3, 60].
As an additional option, stem cells may represent promising alternatives [113]. Specifically, mesenchymal stem cells (MSCs) have the capability to differentiate into a variety of connective tissue cell types, including the bone, cartilage, tendon, muscle, and adipose tissue [27, 108]. These cells may be isolated from various tissues, such as bone marrow, skeletal muscle, synovial membrane, adipose tissue, and umbilical cord blood [6, 7, 27, 52, 71, 99]. Moreover, allogeneic MSCs [25, 108] or induced pluripotent stem (iPS) cells [114, 119] may also be considered. However, there have not been much evidence using these cells forthcoming in terms of preclinical and clinical safety, and thus, further studies with such cells are likely necessary.
In addition, the use of a growth factor or its cocktail (combination), including insulin-like growth factor 1 (IGF-1), transforming growth factor beta 1 (TGF-β1), fibroblast growth factor 2 (FGF-2), and bone morphogenetic proteins (BMP-2, BMP-7), may support tissue maturation for the cartilage [10, 41, 87, 111]. Similar to the cartilage, the bone also possesses a large variety of growth factors that are involved in the regenerative process, including TGF-β; BMP-2, 4, 6, and 7; IGF-1 and 2; and platelet-derived growth factor (PDGF) [91, 97, 100].
On the other hand, some researchers have tested an acellular approach using a scaffold alone [29, 53]. Considering the time and cost-effectiveness, as well as safety issues associated with cell culture, this approach could represent a reasonable strategy in tissue engineering. Scaffolds should be developed to meet requirements such as the recruitment of enough tissue progenitor cells from the host tissue.
Choice of Materials
Several methods have been proposed to develop biphasic scaffolds with the hybridization of two distinct biomaterials, each of which being adequate to integrate with the respective surrounding tissue [67]. Many specific material types have been developed for both cartilage and bone regeneration, which are typically made of biocompatible and biodegradable polymers. For the cartilage layer, natural or synthetic polymer-based scaffolds are commonly used. More recently, scaffold-free implants have been developed and the potential feasibility tested. On the other hand, for a scaffold of the subchondral bone layer, it is important to choose materials with initial mechanical strength, good bone ingrowth, and integration of native surrounding bone. Ceramics, glasses, and metallic materials are commonly used. Also, natural or synthetic polymers, similar to cartilage layer, could be used alone or combined with ceramics [4, 18, 28, 54, 123, 124].
Natural Polymers
The materials of naturally derived polymers could provide a naturally occurring environment for the cells and tissues and thereby potentially facilitate cell proliferation and differentiation [42, 118]. Moreover, natural polymers usually contain specific molecular domains that can support and guide cells at various stages of their development [67, 81]; thus, biological interaction of the scaffold with the host tissue can be enhanced. However, they are, in general, biomechanically weak and less stiff than other materials [81]. As a source of materials, collagen, gelatin, glycosaminoglycan, chitosan, starch, hyaluronic acid, alginate, and bacterial-sourced polymers (hydroxyalkanoates) are commonly used.
Synthetic Polymers
Biodegradable synthetic polymers offer several advantages over other materials for developing scaffolds in tissue engineering. The main advantages are being able to control mechanical properties (i.e., strength and stiffness) and degradation speed [38]. Synthetic polymers are also attractive because they can be fabricated into various shapes with a desired pore according to the speed of cell migration or tissue ingrowth [30]. Moreover, the progression of current techniques such as electrospinning methods and the 3D printer has enabled the simple design and fabrication of scaffolds, which mimic the original tissue structure [61–63]. On the other hand, synthetic polymers have limitations in bioactivity due to their hydrophobic surface not supporting cell attachment and proliferation [14, 89, 101, 106]. Surface treatment with chondroitin sulfate [17], silicate [20], and alkaline [89] could increase hydrophilicity and provide a suitable scaffold for tissue engineering. Also, these polymers, incorporated with growth factors such as TGF-β and BMP, would be helpful and convenient to support cell proliferation and differentiation, stimulating the repair of damaged tissue [93, 94]. As a source of biodegradable synthetic polymers, polyglycolic acid (PGA), poly(D,L-lactic-co-glycolic acid) (PLGA), poly-L-lactic acid (PLLA), polycaprolactone (PCL), and polyethylene glycol (PEG) have been commonly used.
Scaffold-Free Biomaterials
Polymer-based scaffolds have been reported to contribute to good osteochondral repair in vivo [2, 4, 43, 69, 84, 107]. Despite this, there remain several concerns associated with the long-term safety of these constructs due to the involvement of chemical or animal-derived materials. To overcome such potential problems, we have developed a scaffold-free three-dimensional tissue-engineered construct (TEC) composed of MSCs derived from the synovium and ECMs synthesized by the cells (Fig. 23.5a) [6, 7]. The feasibility of the resultant TEC to facilitate cartilage repair was demonstrated in a preclinical large animal model [5, 6, 108], and we have now proceeded clinical studies under the auspices of an approved first-in-man protocol [80]. These TECs are developed without an artificial scaffold, and thus, their implantation could eliminate or minimize the risk of potential side effects induced by extrinsic chemical or biological materials. Furthermore, such TEC are highly adherent to cartilage matrix and secure integration of the TEC until adjacent cartilage tissue is observed following implantation. Therefore, combined constructs of TEC and several materials for the subchondral bone layer may effectively repair an osteochondral lesion with zonal restoration, and TEC could be one of the strong candidates for a cartilage bioimplant. In our animal study, we have demonstrated that the combined bioimplant of TEC and ceramic-based artificial bone significantly accelerated and improved osteochondral repair (Fig. 23.5b, c) [109].
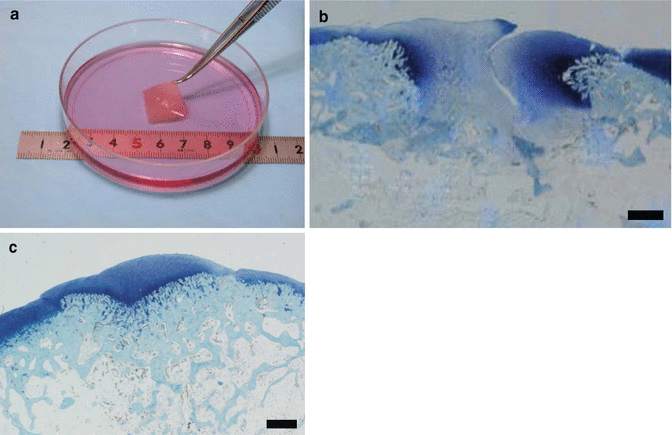

Fig. 23.5
(a) In vitro generated scaffold-free tissue-engineered construct (TEC) composed of MSCs derived from the synovium and ECMs synthesized by the cells. (b, c) Toluidine blue staining of repair tissues in osteochondral defect (b) untreated or (c) treated with the biphasic implant made of TEC and ceramic artificial bone using a rabbit model. The untreated repair tissue showed insufficient cartilage and subchondral bone repair with fibrous or fibrocartilaginous repair at 2 months postoperatively. The repair tissue treated with the implant showed complete osteochondral repair at 2 months postoperatively. Bar = 1 mm
Ceramics and Glasses
Ceramics, such as hydroxyapatite (HA), or other calcium phosphates, such as tricalcium phosphate (TCP), and bioactive glasses, such as Bioglass®, are widely used for bone tissue engineering [45, 70, 117, 121]. These materials promote the formation of a bone-like tissue and enhance integration of the scaffold to the host tissue due to excellent osteoconductivity and osteoinductivity. Also, inclusion of growth factors in the scaffolds may be an interesting concept to explore and contribute to the maturation of bone tissue. Notably, the inclusion of BMP-2 in an HA-based scaffold was reported to promote subchondral bone as well as cartilage repair [117]. On the other hand, these scaffolds have low structural integrity being brittle and unsuitable for applications under mechanical stress, although they exhibit suitable stiffness [81]. The degradation behavior of these scaffolds can be controlled by changes in the porous structures, which can be tailored in terms of their degradation kinetics appropriate for bone tissue engineering. It is also well known that increasing porosity impairs further the mechanical properties of bioceramic scaffolds. This problem can be solved by modifying any porous scaffolds with infiltration or coating by biodegradable polymers [19, 73, 92].
Metallic Materials
Metals are widely used in orthopedic implants such as titanium, titanium alloys, stainless steels, and cobalt-chromium alloy. As an application of osteochondral bone repair, metallic materials withhold the capability of withstanding mechanical loading when used in the subchondral bone layer. On the other hand, the lack of degradation over time and the possibility of wear particle release or corrosion are disadvantages. As one of the examples, porous tantalum was reported to induce subchondral bone growth and showed integration to adjacent host bone in an in vivo rabbit study [11].
Preclinical Study and Clinical Trial
There have been many therapeutic procedures investigated to biologically repair damaged cartilage, some of which are already at the stage of clinical application. On the contrary, considering the higher incidence of OA, which involves subchondral bone pathology, by comparison to isolated chondral injury [8, 24, 26, 40, 88, 126], there is an urgent need to develop novel therapeutic methods for osteochondral repair with clinical relevance. In this regard, the number of animal experiments and clinical trials to treat osteochondral lesions has been recently increased. We focus on the latest preclinical animal studies using large animal and clinical studies.
At first, in order to evaluate the feasibility and safety of new implants with clinical relevance, the selection of appropriate animal models is important. Due to the differences in matrix structure and composition, as well as in the natural osteochondral healing response and technical difficulty in creating the lesions of consistent size and location, the use of small animals such as rabbits, rats, and mice may not be appropriate [1, 21, 95]. Rather, in consideration of clinical relevance, it is preferable to utilize larger animal models, such as pigs, sheep, goats, and horses. Marquass et al. used an MSC-seeded combined implant with a collagen I hydrogel and β-TCP in an ovine osteochondral defect model and showed comparable repair quality to osteochondral autografts in terms of histology and biomechanical testing [69]. Miot et al. prepared engineered cartilage, which was generated from autologous chondrocytes cultured in hyaluronic acid scaffolds of different pre-culture periods, and implanted the engineered cartilage above the hydroxyapatite/hyaluronic acid sponges into goat osteochondral defects. They concluded that 2 weeks pre-culture of engineered cartilage achieved a suitable compromise between tissue maturity and structural/integrative properties of the repair tissue. These data demonstrate that the stage of development of engineered cartilage is an important parameter to be considered in designing cartilage repair strategies [75]. Kon et al. used an aragonite/hyaluronate biphasic scaffold for osteochondral defects in a goat model and showed that mechanical modification with drilled channels in the cartilage phase and impregnation of HA within the coral pores enhanced the scaffold’s cartilage regenerative potential [56]. Schleicher et al. compared two biphasic scaffolds of either hydroxylapatite/collagen or allogenous sterilized bone/collagen and tested their integration in a sheep model. They showed that the latter scaffold proved to be stable and sufficiently integrated in the short term [103]. Sosio et al. treated the osteochondral lesions with a biphasic scaffold made of collagen type I and hydroxyapatite in a pig model, in which they compared the scaffold seeded with autologous chondrocyte with the unseeded scaffold [112]. Surprisingly, they showed that the unseeded scaffold exhibited better macroscopic and histological results than the cell-seeded scaffold. Kon et al. developed an acellular three-gradient multilayer scaffold made of collagen type I and nanoparticles of hydroxyapatite and tested the scaffold with or without autologous chondrocytes in sheep osteochondral defect model. They concluded that the scaffold contributed to the process of the bone and hyaline-like cartilage regeneration, regardless of the use of chondrocytes [54]. Also, they treated 27 patients with chondral or osteochondral lesions using an acellular scaffold [34, 53, 55] and demonstrated the safety and potential clinical benefit of the graded biomimetic osteochondral scaffold in promoting the bone and cartilage tissue with good clinical and magnetic resonance imaging results until the 5-year follow-up. Dhollander et al. treated 27 for cartilage lesions with an acellular osteochondral plug, which is composed of polylactide-co-glycolide copolymer, calcium sulfate, polyglycolide fibers, and surfactant (TruFit plug; Smith and Nephew, Andover, MA) [29]. In this clinical pilot study, a modest clinical improvement became apparent at 12 months of follow-up. Also, MRI data showed no deterioration of the repair tissue. However, 20 % of the patients had persistent clinical symptoms after surgery and had an additional surgery such as removal of the osteochondral plug remnants. The two latter studies were Level IV study, and further studies, which would be compared with conventional treatment such as bone marrow stimulation and osteochondral transplantation, are necessary. Also, in contrast with cell-free scaffolds, no clinical trial using cell-seeded scaffold has been reported, and these studies should be expected in the near future.
Future Directions
The management of OA remains challenging and controversial. Considering the steady progression of tissue engineering and cell-based technologies over the past decade, we may have new therapeutic options for osteochondral repair in clinical practice. In this chapter, we have focused on biomaterial scaffold for osteochondral repair, including the concept, scaffold fabrication, in addition to the selection of cells and materials. There have been many promising scaffolds developed, some of which contribute to good osteochondral repair in vivo. Moreover, some of them are already at the stage of preclinical, large animal studies, as well as clinical trials. In addition, the recent work has been focused on not only investigating the effectiveness of materials or cells but also applying several new concepts and techniques such as mechanical [56], microstructural [30], and local microenvironment modification [94] for the design and fabrication of scaffolds. Therefore, the application of additional new implants to osteochondral lesions could be expected in the near future. On the other hand, the most suitable biomaterials for the cartilage or subchondral bone layers have not been fully investigated, while there are many biomaterials available for osteochondral repair. Therefore, the comparison of these materials should be performed to ultimately determine the ideal material. Further studies will be needed and should be conducted in a methodologically rigorous fashion.
Acknowledgments
This study was supported by a grant from the New Energy and Industrial Technology Development Organization, Japan, Grant-in-Aid for Scientific Research (B), and Japan Society for the Promotion of Science, Japan.
References
1.
Ahern BJ, Parvizi J, Boston R, Schaer TP (2009) Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17(6):705–713. doi:10.1016/j.joca.2008.11.008, S1063-4584(08)00353-1 [pii]PubMed
2.
Ahn JH, Lee TH, Oh JS, Kim SY, Kim HJ, Park IK, Choi BS, Im GI (2009) Novel hyaluronate-atelocollagen/beta-TCP-hydroxyapatite biphasic scaffold for the repair of osteochondral defects in rabbits. Tissue Eng Part A 15(9):2595–2604. doi:10.1089/ten.TEA.2008.0511, 10.1089/ten.TEA.2008.0511 [pii]PubMed
3.
Albrecht C, Schlegel W, Bartko P, Eckl P, Jagersberger T, Vecsei V, Marlovits S (2010) Changes in the endogenous BMP expression during redifferentiation of chondrocytes in 3D cultures. Int J Mol Med 26(3):317–323PubMed
4.
Alhadlaq A, Mao JJ (2005) Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am 87(5):936–944. doi:10.2106/JBJS.D.02104, 87/5/936 [pii]PubMed
5.
Ando W, Fujie H, Moriguchi Y, Nansai R, Shimomura K, Hart DA, Yoshikawa H, Nakamura N (2012) Detection of abnormalities in the superficial zone of cartilage repaired using a tissue engineered construct derived from synovial stem cells. Eur Cell Mater 24:292–307. doi:vol024a21 [pii]
6.
Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N (2007) Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 28(36):5462–5470. doi:10.1016/j.biomaterials.2007.08.030, S0142-9612(07)00672-2 [pii]PubMed
7.
Ando W, Tateishi K, Katakai D, Hart DA, Higuchi C, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N (2008) In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A 14(12):2041–2049. doi:10.1089/ten.tea.2008.0015 PubMed
8.
Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L (2004) Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 32(1):211–215PubMed
9.
Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, Granchi D, Kassem M, Konttinen YT, Mustafa K, Pioletti DP, Sillat T, Finne-Wistrand A (2011) Bone regeneration and stem cells. J Cell Mol Med 15(4):718–746. doi:10.1111/j.1582-4934.2010.01224.x PubMedCentralPubMed
10.
Babensee JE, McIntire LV, Mikos AG (2000) Growth factor delivery for tissue engineering. Pharm Res 17(5):497–504PubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








