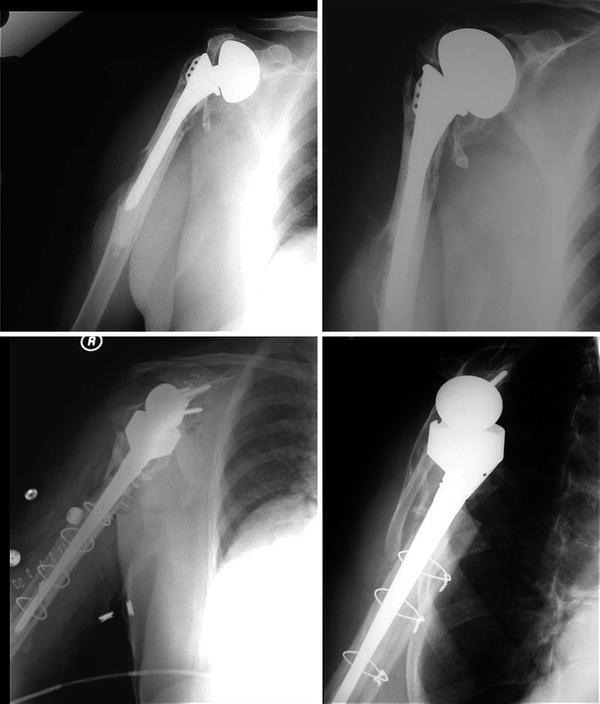
Fig. 22.1
69-year-old male with a painful hemiarthroplasty secondary to subscapularis insufficiency (Top row). This was treated with revision to reverse shoulder arthroplasty with humeral component removal with a successful result (Bottom row)
Instability
Instability following shoulder hemiarthroplasty is a rare but difficult clinical entity to treat. While often associated with massive rotator cuff tear and anterosuperior escape, instability can be disabling for patients due to a variety of etiologies. Anterior capsular and subscapularis insufficiency or glenoid bone loss can contribute to anterior instability after hemiarthroplasty. Severe glenoid retroversion and wear can lead to chronic posterior instability. Failure to restore deltoid tension can lead to inferior subluxation. While capsular or bony operations to correct these issues have been attempted, reverse shoulder arthroplasty has been demonstrated to be most reliable in managing these problems [16].
Revision of Hemiarthroplasty for Fracture
One of the most common current indications for hemiarthroplasty is in proximal humerus fractures that are not amenable to surgical fixation. While hemiarthroplasty for fracture can yield excellent results, when there is failure of tuberosity healing, results are poor [17–19]. Other complications in this setting include implant malposition, tuberosity malunion, rotator cuff insufficiency, and glenoid-sided arthrosis. In these situations, reverse shoulder arthroplasty is commonly the indicated salvage procedure [6, 17, 20].
Infection
Infection is a devastating complication after shoulder arthroplasty and is critical to rule out in any painful shoulder prosthesis. While in some cases infection may be obvious, as in the setting of nonhealing wounds, draining sinus tracts, or purulence, the presentation is, most commonly, subtle. Any patient presenting with a painful hemiarthroplasty should be questioned specifically about wound healing difficulties after surgery, wound revision surgeries or hematoma evacuations, and prolonged wound drainage. In addition, low-grade fevers, chills, and night sweats are uncommon, but occasionally seen in the setting of low-grade infection. In our experience, humeral component loosening is often associated with an infection. The complete workup and diagnosis of infection is controversial and beyond the scope of this chapter.
Treatment of prosthetic infection is a complex problem, with significant national and international variation in algorithms for management. The expanded use of the reverse shoulder arthroplasty has improved the flexibility and success in managing these difficult problems. In the setting of chronic infection, and in the face of multiple revision surgeries, the rotator cuff is frequently attenuated or torn. For this reason, the majority of implants placed in the setting of infection, whether treated through one-stage or two-stage revision, are reverse total shoulder arthroplasties [21, 22].
Preferred Method of Treatment
Preoperative Evaluation
When the decision has been made to revise the painful hemiarthroplasty to a reverse total shoulder arthroplasty, there are a number of factors to take into consideration that may have a dramatic impact on the operative plan and techniques. These factors include tuberosity integrity, glenoid bone stock and version, nerve integrity (especially in cases with a fracture or dislocation history), prior surgical incisions/approaches, implant type and method of fixation, status of the contralateral extremity, and patient comorbidities. Preoperative workup may include history and physical examination, adequate plain radiographs (anteroposterior views in internal and external rotation, scapular Y view, and axillary view), CT scan, EMG and nerve conduction velocity, ESR and CRP, joint aspiration, and medical consultation. In this section, we will assume that revisions are being performed for aseptic reasons or that preexisting infection has been successfully irradicated.
An important consideration is the approach used for the index procedure. In patients with hemiarthroplasty through a superior approach, a separate deltopectoral approach is routinely necessary to allow extensibility in the setting of difficult stem extraction, cement extraction, and proximal bone loss. Whenever possible, operative reports and implant specifications should be obtained in order to have stem-specific extraction devices available. In the setting of convertible stems, reverse epiphysis components may need to be specifically ordered for conversion to reverse shoulder arthroplasty. When there is bony deformity or bone loss, a computed tomography (CT) scans are often indicated to assess glenoid version and bone stock. Three-dimensional reconstructions of the scapula with subtraction of the humerus can allow for a complete assessment of deformity and bone loss. Despite the introduction of metal suppression software, metal artifact can often limit the information obtained with CT scans. Patient positioning in the scanner can help decrease metal artifact [23]. This type of preoperative planning can allow for more accurate placement of the reverse component baseplate, determine the need for bone grafting, and allow for selection of the most appropriate baseplate design (i.e., long pegs and multiple screws). In addition, it can allow better assessment of proximal humeral bony architecture and tuberosity position.
It is also important to have in the operating room all potential equipment necessary for the revision. This includes a c-arm, ultrasonic cement removal device, revision instruments, fixation cables, plates, and screws, structural and cancellous allograft, antibiotic impregnated bone cement, and all necessary implants with their accompanying instruments. In addition, one may consider intraoperative nerve monitoring in cases with known nerve injury or severe stiffness as well as nerve stimulators to help in identifying nerves and preventing nerve injury.
Exposure
An extended deltopectoral approach is utilized in nearly all cases of revision hemiarthroplasty to reverse shoulder replacement. The skin incision begins superior and medial to the tip of the coracoid process and extends to the deltoid tuberosity. The surgeon should be prepared to extend this into an anterolateral approach to the humerus in the case of intraoperative fracture or cement extrusion. Scarring is usually present at every level and must be cleared to allow mobilization of all tissue planes from superficial to deep. Once the incision has been taken down to the level of the deltoid and pectoralis major, the deltopectoral interval is identified by the cephalic vein, if one is present, or the tip of the coracoid. The deltopectoral interval is then developed to the level of the deltoid tuberosity. The pectoralis major is usually adhered to the conjoined tendon and must be released. This dissection is started at the tip of the coracoid and extends distally, superficial to the conjoined tendon, until there is complete separation of the pectoralis major from the underlying conjoined tendon. It is also important to release the superior border of the pectoralis tendon from the distal portion of the conjoined tendon. A small portion of the pectoralis tendon may be released. The musculocutaneous nerve may be quite lateral in revision situations and care should be taken in dissecting the upper border of the pectoralis tendon from the distal portion of the conjoined tendon. A nerve stimulator may be useful in confirming the presence of the musculocutaneous nerve.
The deltoid must next be freed from the underlying humerus. The safest way to accomplish this is to start proximally and distally and work toward the axillary nerve. It is also useful to place the arm in 30°–40° of abduction and slight flexion to relax the deltoid. The interval between the distal deltoid tendon and the humeral shaft is usually easily identified at the level of the midportion of the pectoralis major insertion. Moreover, at this level, the dissection is well below the level of the axillary nerve. This interval is developed and a blunt human retractor is placed between the deltoid and the humeral shaft. Dissection is then taken proximally to a point 2–3 cm inferior to the surgical neck. This should be well below the level of the axillary nerve, but one should proceed with caution the more proximal the dissection is taken. The proximal portion of the anterior deltoid is retracted superiorly with progressive separation of the deltoid from the underlying humerus. The leading edge of the coracoacromial ligament is then identified, and another blunt human retractor is placed under the leading edge of the most medial extent of the coracoacromial ligament. A path for the retractor can usually be made by first pushing through any scar with a curved mayo scissor or small Cobb elevator. The adhesions in the subacromial space are then sharply released from medial to lateral. The humerus is then internally rotated, and the dissection is taken distally toward the surgical neck. As the axillary nerve is approached, blunt dissection should be used. Once the adhesions between the deltoid and underlying humerus, within the subacromial space, and between the pectoralis major and conjoined tendon have been released, the deltoid is retracted laterally and the pectoralis major is retracted medially using a Kolbel self-retaining retractor and a blunt homan retractor is placed superiorly within the subacromial space.
The plane between the conjoined tendon and the underlying subscapularis is also often obliterated with scar. The safest way to develop this layer is to start superiorly, just inferior to the tip of the coracoid. The dissection is started laterally, at the lateral edge of the conjoined tendon, and taken medially. When the base of the coracoid is reached, dissection should be blunt and careful as the axillary artery and vein can be closer to the surgical field than usual. When a small space within the scar has been created superiorly, digital palpation can be utilized to identify the axillary nerve. The dissection can then be carried distally to the inferior aspect of the subscapularis. The conjoined tendon is then retracted medially with the medial arm of the self-retaining retractor. Care should be taken to avoid excessive force on the medial retraction, especially if the musculocutaneous nerve has been identified more laterally than usual (Fig. 22.2). In some cases, the axillary nerve cannot be definitely identified because of the presence of severe scarring or substantial subscapularis deficiency. Under these circumstances, it may be useful to reflect the anterior soft tissue envelope (or what is left of it) from the humerus, staying directly on bone, and identifying the nerve after placing traction sutures in the reflected anterior soft tissue envelope. A nerve stimulator may also facilitate axillary nerve identification and protection. Rarely, detachment of the conjoined tendon may be required.
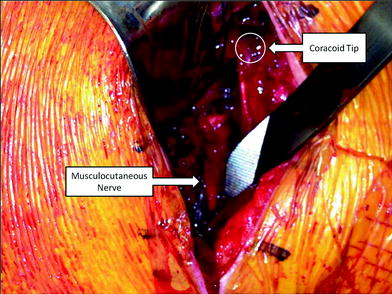

Fig. 22.2
Intraoperative photograph of a revision right shoulder hemiarthroplasty to reverse total shoulder arthroplasty. The musculocutaneous nerve can be seen scarred into a far lateral position, bringing it into the surgical field. Care must be taken in the revision setting to identify and protect neurovascular structures that may be encased in scar
With the conjoined tendon retracted medially, the intact soft tissue envelope consisting of the subscapularis and capsule can be identified. Usually, the anterior humeral vessels are absent but if they are present, they should be clamped and coagulated or ligated. In addition, if the biceps is still present within the bicipital groove, it is tenodesed to the upper border of the pectoralis major and resected proximal to the tenodesis site. The subscapularis may be reflected through the use of an intratendinous incision, tendon reflection from bone, or a lesser tuberosity osteotomy. If the subscapularis is in good quality, has a completely intact insertion to the lesser tuberosity, and passive external rotation is to at least 10°, the senior author prefers to use a thin (5 mm) lesser tuberosity osteotomy. The subscapularis is reflected with the lesser tuberosity, leaving the capsule attached to the humerus. If the layer between the subscapularis and capsule cannot be safely developed, the capsule and subscapularis are reflected in a single layer. In almost all other cases, the tendon is detached from the lesser tuberosity as far laterally as possible and subscapularis and capsule are reflected in a single layer. In either case, the inferior capsule is released from the humerus past the 6 o’clock position and up to the insertion of the teres minor. This requires slight flexion, adduction, and external rotation of the humerus. Care should be taken to protect the axillary nerve.
The humerus is then delivered into the wound with simultaneous adduction, extension, and external rotation. The humeral head is removed, and any soft tissue membrane that formed between the head and the humerus is excised. If any portion of the supraspinatus is intact, it is released and excised. Leaving an intact supraspinatus may impede reduction, and the vector of pull is altered such that it is unlikely to provide benefit if left in place. In the presence of prior fracture, an assessment of greater tuberosity position and healing is made. If the greater tuberosity is un-united, it is released from the humerus, the deep surface is freshened and two heavy nonabsorbable sutures are placed through the bone tendon junction for later reattachment. If the humerus is so severely malunited that glenoid exposure or subsequent reduction of the humerus will be compromised, it is osteotomized. The arm is then placed in slight abduction, extension, and external rotation using either a mechanical arm holder or padded mayo stand and a Fukuda retractor is placed within the joint to retract the humerus posteriorly.
If the subscapularis was reflected separate from the capsule, the anterior and inferior capsules are excised. The remaining capsule is released. If the subscapularis and capsule were reflected in a single layer, the capsule is released from the glenoid 360°. In addition, the subscapularis is released circumferentially, paying particular attention to any adhesions between the superior border of the subscapularis and the base of the coracoid. Dissection on the superficial surface of the subscapularis should not be taken medial to the base of the coracoid to avoid denervation of the subscapularis. A reverse double-pronged Bankart retractor is placed on the anterior neck of the scapula and a blunt homan retractor is placed posterosuperiorly, superior to the Fukuda retractor. The labrum is excised circumferentially so that the most inferior extent of the glenoid can be visualized. In addition, in cases of prolonged proximal humeral migration, the inferior capsule can be extremely thick and may interfere with inferior glenoid exposure. Under these circumstances, the inferior capsule can be excised. A nerve stimulator can help to identify and protect the axillary nerve.
Glenoid Baseplate Options/Glenosphere Placement
When choosing reverse arthroplasty devices, several glenoid baseplate options are available that may provide advantages and disadvantages depending on the patient characteristics. Separate chapters in this text expound on the specific design advantages of different components. In general terms, however, the surgeon should consider baseplate ingrowth surfaces, number of screws available, position of screws (circumferential and/or central screws), ability to lock screws, and medial or lateral offset designs. Consideration may be given to choosing a more lateralized design or lateralizing the component with a bone graft (BioRSA) in the setting of revision of hemiarthroplasty, especially with significant glenoid wear. Because there is no humeral head available, options for BioRSA include iliac crest autograft or allograft [24]. As eccentric wear is commonly seen superiorly, the bone graft can be shaped to preferentially augment the superior glenoid, allowing for neutral or inferior inclination.
The baseplate should be placed at the inferior-most margin of the glenoid so that the glenosphere overhangs inferior to the native glenoid by 2–4 mm. This minimizes the chances of postoperative notching, particularly if a glenoid system is used that puts the center of rotation on the glenoid surface (i.e., original Grammont design). Lateralizing the center of rotation also decreases the likelihood of notching and some systems have the ability to variably lateralize the center of rotation. It is important for the surgeon to be familiar with the system being used in order to avoid any pitfalls and take advantage of any unique design features. Glenosphere size should be individualized to the patient based on the patient size, soft tissue integrity, and stability. In general, a 42-mm sphere is used in large males and a 36- or 38-mm sphere is used in small females. Although using the largest sphere possible in all patients may improve stability, it is possible to overstuff the joint, even with a reverse. If fixation of the baseplate is adequate and the soft tissues that remain are of reasonable quality, it is reasonable to place the real glenosphere at this time. If there is any question about stability, a trial sphere is placed.
Humeral Revision
Attention is next turned to the humerus. Most cementless stems, even if they are proximally porous coated, can be removed without splitting the humerus (episiotomy) or making a window. Small osteotomes are passed distally from the top of the stem, circumferentially, to separate the proximal portion of the stem from the humeral cancellous bone. If the stem has a large collar or the proximal porous coating extends into the proximal diaphysis, a small, v-shaped window can be made at the medial calcar so that the bone–prosthesis interface can be accessed. It is also important to identify and remove or incise any sutures that were previously placed between the humerus and humeral stem as attempting to remove the stem without disrupting the sutures may result in proximal humeral fracture. The stem can almost always then be removed with either implant-specific extractors or a tamp placed at the medial portion of the stem and a mallet.
Cemented stems are usually much more difficult to remove than uncemented stems, particularly if they have a porous coating that was incorporated into the cement mantle. Two main categories of humeral osteotomies have been described: humeral windows and “vertical humeral osteotomies” (aka humeral splits or episiotomies). Clinical success has been documented with either approach [25–28]. If the stem is completely smooth, especially if it is tapered distally, it can often be removed without an episiotomy or a window. Osteotomes are passed, and previous sutures are removed as described above for cementless stems. An attempt is then made to mallet the stem out with either device-specific extractors or a medially placed tamp. If this fails, an episiotomy is made the length of the stem approximately 1 cm lateral to the bicipital groove. The split in the humerus is initially made with a saw starting superiorly and extending to the tip of the cement mantle, which can be identified using c-arm. A 2-mm drill hole is placed at the distal extent of the saw cut to avoid distal propagation. A wide, straight osteotome is then tapped into the entire length of the saw cut down to the level of the prosthesis. If there is no cement in the porous coating of the implant, the stem is almost always easily removed using a medially placed tamp or device-specific extractor and a mallet. When the stem has been removed, the next step is to place cables around the humerus to stabilize the episiotomy. Alternatively, cables can be placed prior to the episiotomy and tightened afterward.
If the episiotomy fails to loosen the stem enough for extraction, a humeral window is required. In the senior author’s practice, this occurs approximately 5–10 % of the time and is usually associated with a porous-coated stem that has been cemented. Starting at the drill hole that was placed at the distal extent of the episiotomy, a microsagittal saw is used to make a saw cut in an anterior direction, perpendicular to the episiotomy. This anterior saw cut proceeds up to the medial humeral cortex, usually 15–20 mm. At its most medial extent, a second 2-mm drill hole is placed to minimize the possibility of medial or distal propagation. With the humerus subperiosteally exposed and the medial soft tissues protected, the microsagittal saw is used to make a medial cut that is parallel to the episiotomy, starting distally and working proximally. The cut is stopped when the inferior border of the pectoralis major is encountered. The saw is then moved superior to the pectoralis major and is used to continue the cut to the superior extent of the medial humerus. A small straight osteotome is then placed in the cut inferior to the pectoralis major insertion and tapped gently with the mallet to connect the superior and inferior cuts. A large straight osteotome is then placed into the lateral episiotomy and rotated carefully to displace the humeral window. The stem can then usually be removed. Any portion of the cement mantle that is loose is removed. In addition, the cement plug is either removed or perforated with an ultrasonic cement remover under direct vision. It is important to thoroughly irrigate and take frequent breaks when using the ultrasonic cement removal. Heat from the device can cause nerve injury (commonly radial nerve) and humeral bone necrosis.
The next step is to replace the window and fix it with cables. If the distal extent of the window is distal to the deltoid insertion, the radial nerve may be at risk during cable placement. Under these circumstances, the skin incision should be extended distally and the anterolateral surface of the humerus should be exposed. In addition, if the humeral diaphysis is of poor quality, medial and or lateral humeral or tibial strut allografts may be considered (Fig. 22.3). If there has been an unintended fracture of the humeral diaphysis, a formal anterolateral approach to the humerus should be made, taking care to protect the radial nerve, and the fracture fixed.