Chapter 5 Conventional Intracardiac Mapping Techniques
Activation Mapping
Fundamental Concepts
The main value of intracardiac and surface ECG tracings consists of the comparative timing of electrical events and the determination of the location and direction of impulse propagation. Additionally, electrogram morphology can be of significant importance during mapping. Interpretation of recorded electrograms is fundamental to the clinical investigation of arrhythmias during electrophysiological (EP) studies. Establishing electrogram criteria, which permit accurate determination of the moment of myocardial activation at the recording electrode, is critical for construction of an area map of the activation sequence. Bipolar recordings are generally used for activation mapping. Unipolar recordings are used to supplement the information obtained from bipolar recordings. The differences in unipolar and bipolar recordings can be used to assist in mapping by simultaneously recording bipolar and unipolar signals from the mapping catheter.1
Unipolar Recordings
Timing of Local Activation
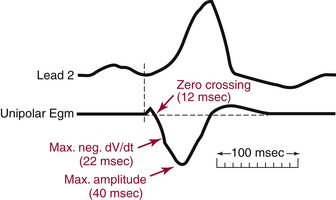
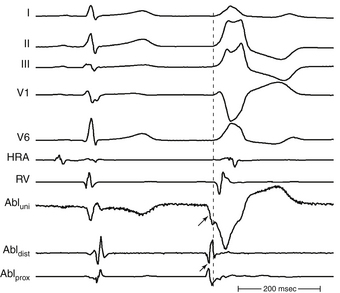
The major component of the unipolar electrogram allows determination of the local activation time, although there are exceptions. The point of maximum amplitude, the zero crossing, the point of maximum slope (maximum first derivative), and the minimum second derivative of the electrogram have been proposed as indicators of underlying myocardial activation (Fig. 5-1). The maximum negative slope (i.e., maximum change in potential, dV/dt) of the signal coincides best with the arrival of the depolarization wavefront directly beneath the electrode because the maximal negative dV/dt corresponds to the maximum sodium channel conductance. Using this fiducial point, errors in determining the local activation time as compared with intracellular recordings have typically been less than 1 millisecond. This is true for filtered and unfiltered unipolar electrograms.1,2
Direction of Local Activation
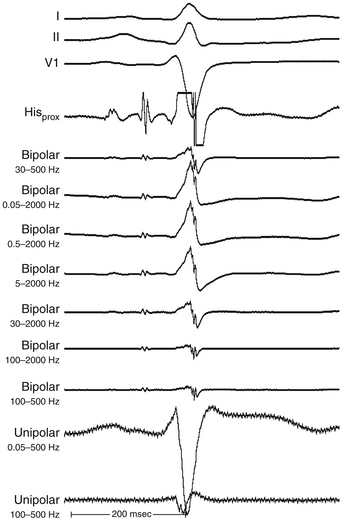
The morphology of the unfiltered unipolar recording indicates the direction of wavefront propagation. By convention, the mapping electrode that is in contact with the myocardium is connected to the positive input of the recording amplifier. In this configuration, positive deflections (R waves) are generated by propagation toward the recording electrode, and negative deflections (QS complexes) are generated by propagation away from the electrode (Figs. 5-2 and 5-3). If a recording electrode is at the source from which all wavefronts propagate (at the site of initial activation), depolarization will produce a wavefront that spreads away from the electrode, thus generating a monophasic QS complex. It is also important to recognize that a QS complex can be recorded when the mapping electrode is not in contact with the myocardium, but is floating in the cavity. In that situation, the initial negative slope of the recording is typically slow, suggesting that the electrogram is a far-field signal, generated by tissue some distance from the recording electrode.1 Filtering at higher corner frequencies (e.g., 30 Hz) alters the morphology of the signal, so that the morphology of the unipolar electrogram is no longer an indication of the direction of wavefront propagation, and the presence or absence of a QS complex cannot be used to infer proximity to the site of earliest activation (Fig. 5-4).2
Disadvantages of Unipolar Recordings
The major disadvantage of unipolar recordings is that they have poor signal-to-noise ratio and contain substantial far-field signal generated by depolarization of tissue remote from the recording electrode. Therefore, distant activity can be difficult to separate from local activity. This is especially true when recording from areas of prior myocardial infarction (MI), where the fractionated ventricular potentials are ubiquitous and it is often impossible to select a rapid negative dV/dt when the entire QS potential is slowly inscribed—that is, cavity potential.2 Another disadvantage is the inability to record an undisturbed electrogram during or immediately after pacing. This is a significant disadvantage when entrainment mapping is to be performed during activation mapping, because recording of the return tachycardia complex on the pacing electrode immediately after cessation of pacing is required to interpret entrainment mapping results.1
Bipolar Recordings
Timing of Local Activation
Algorithms for detecting local activation time from bipolar electrograms have been more problematic, partly because of generation of the bipolar electrogram by two spatially separated recording poles. In a homogeneous sheet of tissue, the initial peak of a filtered (30 to 300 Hz) bipolar signal, the absolute maximum electrogram amplitude, coincides with depolarization beneath the recording electrode, appears to correlate most consistently with local activation time, and corresponds to the maximal negative dV/dt of the unipolar recording (see Fig. 5-3).2 However, in the case of complex multicomponent bipolar electrograms, such as those with marked fractionation and prolonged duration seen in regions with complex conduction patterns (e.g., in regions of slow conduction in macroreentrant atrial tachycardia [AT] or ventricular tachycardia [VT]), determination of local activation time becomes problematic, and the decision about which activation time is most appropriate needs to be made in the context of the particular rhythm being mapped. To acquire true local electrical activity, a bipolar electrogram with an interelectrode distance of less than 1 cm is desirable. Smaller interelectrode distances record increasingly local events (as opposed to far-field). Elimination of far-field noise is usually accomplished by filtering the intracardiac electrograms, typically at 30 to 500 Hz.1,2
Direction of Local Activation
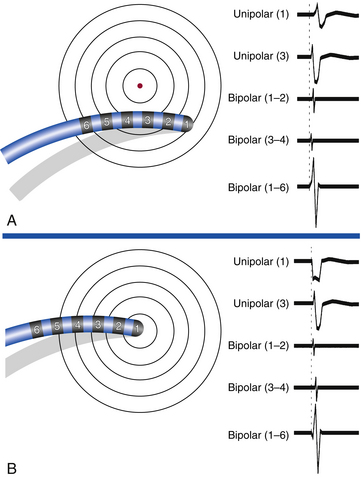
The morphology and amplitude of bipolar electrograms are influenced by the orientation of the bipolar recording axis to the direction of propagation of the activation wavefront. A wavefront that is propagating in the direction exactly perpendicular to the axis of the recording dipole produces no difference in potential between the electrodes and hence no signal.1,2 However, the direction of wavefront propagation cannot be reliably inferred from the morphology of the bipolar signal, although a change in morphology can be a useful finding. For example, when recording from the lateral aspect of the cavotricuspid isthmus during pacing from the coronary sinus (CS), a reversal in the bipolar electrogram polarity from positive to negative at the ablation line indicates complete isthmus block. Similarly, if bipolar recordings are obtained with the same catheter orientation parallel to the atrioventricular (AV) annulus during retrograde bypass tract (BT) conduction, an RS configuration electrogram will be present on one side of the BT, where the wavefront is propagating from the distal electrode toward the proximal electrode, and a QR morphology electrogram will be present on the other side, where the wavefront is propagating from the proximal electrode toward the distal electrode (Fig. 5-5).
Disadvantages of Bipolar Recordings
In contrast to unipolar signals, the direction of wavefront propagation cannot be reliably inferred from the morphology of the bipolar signal. Furthermore, bipolar recordings do not allow simultaneous pacing and recording from the same location. To pace and record simultaneously in bipolar fashion at endocardial sites as close together as possible, electrodes 1 and 3 of the mapping catheter are used for bipolar pacing, and electrodes 2 and 4 are used for recording. The precision of locating the source of a particular electrical signal depends on the distance between the recording electrodes, because the signal of interest can be beneath the distal or proximal electrode (or both) of the recording pair.1
Mapping Procedure
Selection of the Electrical Reference Point
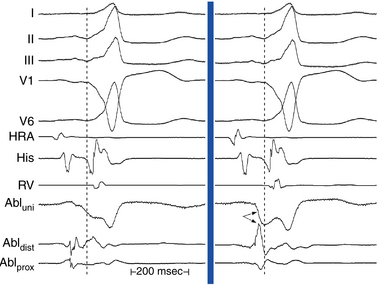
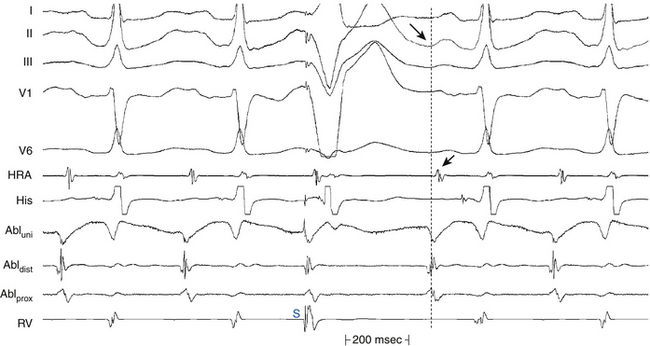
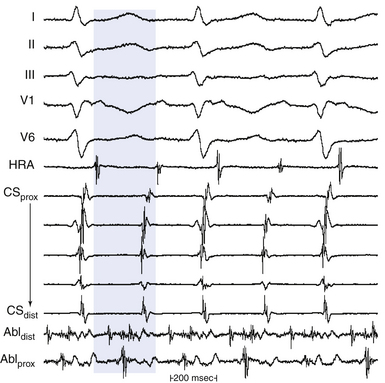
Local activation times must be relative to some external and consistent fiducial marker, such as the onset of the P wave or QRS complex on the surface ECG or a reference intracardiac electrode. For VT, the QRS complex onset should be assessed using all surface ECG leads to search for the lead with the earliest QRS onset. This lead should then be used for subsequent activation mapping. Similarly, the P wave during AT should be assessed using multiple ECG leads and choosing the one with the earliest P onset. However, determining the onset of the P wave can be impossible if the preceding T wave or QRS is superimposed. To facilitate visualization of the P wave, a ventricular extrastimulus (VES) or a train of ventricular pacing can be delivered to anticipate ventricular activation and repolarization and permit careful distinction of the P wave onset (Fig. 5-6). After determining P wave onset, a surrogate marker, such as a right atrial (RA) or CS electrogram indexed to the P wave onset, where it is clearly seen, can be used rather than the P wave onset.
Defining the Goal of Mapping
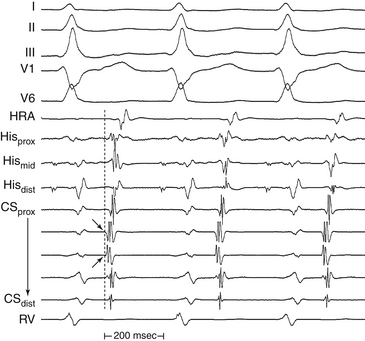
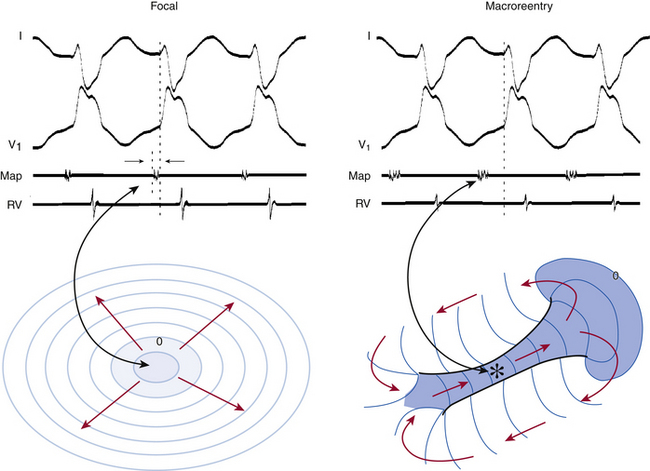
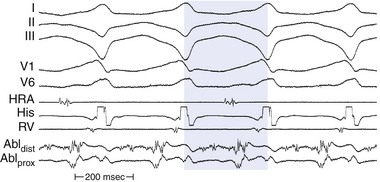
Determination of the mechanism of the tachycardia (focal versus macroreentrant) is essential to define the goal of activation mapping. For focal tachycardias, activation mapping entails localizing the site of origin of the tachycardia focus. This is reflected by the earliest presystolic activity that precedes the onset of the P wave (during focal AT) or QRS (during focal VT) by an average of 10 to 40 milliseconds, because only this short amount of time is required after the focus discharges to activate enough myocardium and begin generating a P wave or QRS complex (Fig. 5-7). For mapping macroreentrant tachycardias, the goal of mapping is identification of the critical isthmus of the reentrant circuit, as indicated by finding the site with a continuous activity spanning diastole or with an isolated mid-diastolic potential (see Fig. 5-7).
Epicardial Versus Endocardial Mapping
Another epicardial mapping technique utilizes a subxiphoid percutaneous approach for accessing the epicardial surface. This technique has become an important adjunctive strategy to ablate a diverse range of cardiac arrhythmias including cardiomyopathic VT, BTs, atrial fibrillation, and idiopathic VTs. Transthoracic epicardial mapping and ablation are seeing increasingly wider application, especially in patients with scar-related VT, in whom more reentrant circuits with vulnerable isthmuses are on the epicardial surface. The same fundamental principles of activation mapping are used for both endocardial mapping and epicardial mapping.3–7
Mapping Focal Tachycardias
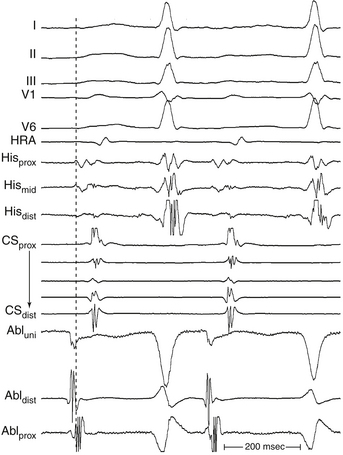
The goal of activation mapping of focal tachycardias (automatic, triggered activity, or microreentrant) is identifying the site of origin, defined as the site with the earliest presystolic bipolar recording in which the distal electrode shows the earliest intrinsic deflection and QS unipolar electrogram configuration (Figs. 5-8 and 5-9). Local activation at the site of origin precedes the onset of the tachycardia complex on the surface ECG by an average of 10 to 40 milliseconds. Earlier electrograms occurring in mid-diastole, as in the setting of macroreentrant tachycardias, are not expected and do not constitute a target for mapping.1
Endocardial activation mapping of focal tachycardias can trace the origin of activation to a specific area, from which it spreads centrifugally. There is generally an electrically silent period in the tachycardia cycle length (CL) that is reflected on the surface ECG by an isoelectric line between tachycardia complexes. Intracardiac mapping shows significant portions of the tachycardia CL without recorded electrical activity, even when recording from the entire cardiac chamber of tachycardia origin. However, in the presence of complex intramyocardial conduction disturbances, activation during focal tachycardias can extend over a large proportion of the tachycardia CL, and conduction spread can follow circular patterns suggestive of macroreentrant activation.2,4
Technique of Activation Mapping of Focal Tachycardias
Initially, one should seek the general region of the origin of the tachycardia as indicated by the surface ECG. In the EP laboratory, additional data can be obtained by placing a limited number of catheters within the heart in addition to the mapping catheter or catheters; these catheters are frequently placed at the right ventricular apex, His bundle region, high RA, and CS. During initial arrhythmia evaluation, recording from this limited number of sites allows a rough estimation of the site of interest. Mapping simultaneously from as many sites as possible greatly enhances the precision, detail, and speed of identifying regions of interest.1
Local activation time is then determined from the filtered (30 to 300 Hz) bipolar signal recorded from the distal electrode pair on the mapping catheter; this time is determined and compared with the timing reference (fiducial point). The distal pole of the mapping catheter should be used for mapping the earliest activation site because it is the pole through which RF energy is delivered. Activation times are generally measured from the onset of the first rapid deflection of the bipolar electrogram to the onset of the tachycardia complex on the surface ECG or surrogate marker (see Fig. 5-6). Using the onset (rather than the peak or nadir) of a local bipolar electrogram is preferable because it is easier to determine reproducibly, especially when measuring heavily fractionated, low-amplitude local electrograms.4
Once the site with the earliest bipolar signal is identified, the unipolar signal from the distal ablation electrode should be used to supplement bipolar mapping.2 The unfiltered (0.05 to 300 Hz) unipolar signal morphology should show a monophasic QS complex with a rapid negative deflection if the site was at the origin of impulse formation (see Figs. 5-8 and 5-9). However, the size of the area with a QS complex can be larger than the tachycardia focus, exceeding 1 cm in diameter. Thus, a QS complex should not be the only mapping finding used to guide ablation. Successful ablation is unusual, however, at sites with an RS complex on the unipolar recording, because these are generally distant from the focus (see Fig. 5-2). Concordance of the timing of the onset of the bipolar electrogram with that of the filtered or unfiltered unipolar electrogram (with the rapid downslope of the S wave of the unipolar QS complex coinciding with the initial peak of the bipolar signal) helps ensure that the tip electrode, which is the ablation electrode, is responsible for the early component of the bipolar electrogram. The presence of ST elevation on the unipolar recording and the ability to capture the site with unipolar pacing are used to indicate good electrode-tissue contact.4
Mapping Macroreentrant Tachycardias
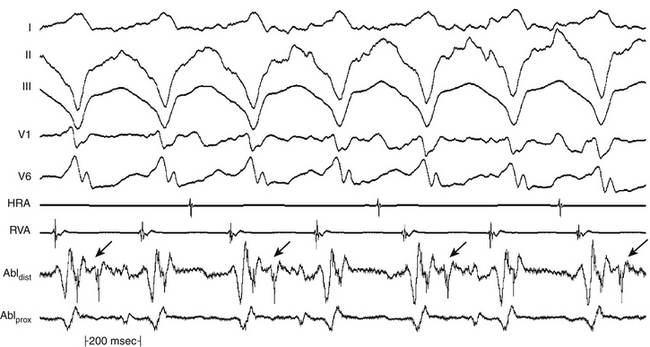
The main goal of activation mapping of macroreentrant tachycardias (e.g., post-MI VT, macroreentrant AT) is identification of the isthmus critical for the macroreentrant circuit.8 The site of origin of a tachycardia is the source of electrical activity producing the tachycardia complex. Although this is a discrete site of impulse formation in focal rhythms, during macroreentry it represents the exit site from the diastolic pathway (i.e., from the critical isthmus of the reentrant circuit) to the myocardium that gives rise to the ECG deflection. During macroreentry, an isthmus is defined as a corridor of conductive myocardial tissue bounded by nonconductive tissue (barriers) through which the depolarization wavefront must propagate to perpetuate the tachycardia. These barriers can be scar areas or naturally occurring anatomical or functional (present only during tachycardia, but not in sinus rhythm) obstacles. The earliest presystolic electrogram closest to mid-diastole is the most commonly used definition for the site of origin of the reentrant circuit. However, recording continuous diastolic activity or bridging of diastole at adjacent sites, or both, or mapping a discrete diastolic pathway is more specific. Therefore, the goal of activation mapping during macroreentry is finding the site or sites with continuous activity spanning diastole or with an isolated mid-diastolic potential. Unlike focal tachycardias, a presystolic electrogram preceding the tachycardia complex by 10 to 40 milliseconds is not adequate in defining the site of origin of a macroreentrant tachycardia (Figs. 5-10 and 5-11; see also Fig. 5-7).2,4
However, identification of critical isthmuses is often challenging. The abnormal area of scarring, where the isthmus is located, is frequently large and contains false isthmuses (bystanders) that confound mapping. Additionally, multiple potential reentry circuits can be present, giving rise to multiple different tachycardias in a single patient. Furthermore, in abnormal regions such as infarct scars, the tissue beneath the recording electrode can be small relative to the surrounding myocardium outside the scar; thus, a large far-field signal can obscure the small local potential. For this reason, despite the limitations of bipolar recordings, these recordings are preferred in scar-related VTs because the noise is removed and high-frequency components are more accurately seen. Unipolar recordings are usually of little help when mapping arrhythmias associated with regions of scar, unless the recordings are filtered to remove far-field signal. Much of the far-field signal in a unipolar recording consists of lower frequencies than the signal generated by local depolarization because the high-frequency content of a signal diminishes more rapidly with distance from the source than the low-frequency content. Therefore, high-pass filtering of unipolar signals (at 30 or 100 Hz) is generally used when mapping scar-related arrhythmias to reduce the far-field signal and improve detection of lower amplitude local signals from abnormal regions.1
Although activation mapping alone is usually inadequate for defining the critical isthmus of a macroreentrant tachycardia, it can help guide other mapping modalities (e.g., entrainment or pace mapping, or both) to the approximate region of the isthmus.4,8
Continuous Activity
Theoretically, if reentry were the mechanism of the tachycardia, electrical activity should occur throughout the tachycardia cycle. For example, in macroreentrant AT, the recorded electrical activity at different locations in the atrium should span the tachycardia CL (see Fig. 5-10).
For macroreentrant VT, conduction during diastole is extremely slow and is in a small area so that it is not recorded on the surface ECG. The QRS complex is caused by propagation of the wavefront from the exit of the circuit from that isthmus to the surrounding myocardium. After leaving the exit of the isthmus, the circulating wavefront propagates through a broad path (loop) along the border of the scar, back to the entrance of the isthmus (see Fig. 5-7).8 Continuous diastolic activity is likely to be recorded only if the bipolar pair records a small circuit; if a large circuit is recorded (i.e., the reentrant circuit is larger than the recording area of the catheter, the catheter is not covering the entire circuit, or both), nonholodiastolic activity will be recorded. In such circuits, repositioning of the catheter to other sites may allow visualization of what is termed bridging of diastole; electrical activity in these adjacent sites spans diastole.2,4
All areas from which diastolic activity is recorded are not necessarily part of the reentrant circuit. Such sites can reflect late activation and may not be related to the tachycardia site of origin. Analysis of the response of these electrograms to spontaneous or induced changes in tachycardia CL is critical in deciding their relationship to the tachycardia mechanism. Additionally, electrical signals that come and go throughout diastole should not be considered continuous (Fig. 5-12). For continuous activity to be consistent with reentry, it must be demonstrated that such electrical activity is required for initiation and maintenance of the tachycardia, so that termination of the continuous activity, either spontaneously or following stimulation, without affecting the tachycardia, would exclude such continuous activity as requisite for sustaining the tachycardia. It is also important to verify that an electrogram that extends throughout diastole is not just a broad electrogram whose duration equals the tachycardia CL. This can be achieved by analyzing the local electrogram during pacing at a CL comparable to tachycardia CL; if pacing produces continuous diastolic activity in the absence of tachycardia, the continuous electrogram has no mechanistic significance. Furthermore, the continuous activity should be recorded from a circumscribed area, and motion artifact should be excluded.4,8
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree