30 Key Points 1. Overall therapeutic goals are discussed, including neuroprotection, neural repair, and functional recovery. 2. Necessary steps in the preclinical translation of a discovery to human application are outlined. 3. The differences between clinical trial phases are highlighted. 4. Potential confounding factors that can alter the outcomes of a clinical trial are described. 5. The guiding principles for the development of clinical trial protocols are listed. Over the past few years there has been increasing interest in the translation of experimental therapeutic interventions to improve functional outcomes after spinal cord injury (SCI). The number of reported successes using preclinical animal models have been substantial and encouraged the development of several clinical trial programs. The overall goals for the treatment of SCI might be summarized as a series of temporally overlapping targets or goals to preserve or improve functional capacity (Table 30.1). At this time there is limited human efficacy data; thus, the timelines for effective neuroprotective, reparative, regenerative, and recovery treatments are necessarily vague. It is not the intent of this chapter to review the mechanisms underlying experimental treatment strategies. Instead, the focus is on the principled pathway for translation of discoveries, as well as the factors that may influence outcomes or confound the accurate interpretation of clinical trial results. Protection strategies are directed against the mechanical, pathological, and inflammatory effects on spinal cord tissues immediately following the primary injury and throughout the first few weeks when secondary cell death processes are most active. Surgical decompression of the cord and mechanical stabilization of the spine constitute standard acute care to ensure the spinal column does not impinge on the cord and cause further damage. The maintenance of adequate vascular perfusion of the spinal cord is also a current standard of care, whereas the reduction of spinal edema and minimization of secondary cell death (e.g., apoptosis) are research goals for acute treatments.1,2 Table 30.1 Overall Goals for Improving Functional Capacity after Spinal Cord Injury
Considerations for the Initiation and Conduct of Spinal Cord Injury Clinical Trials
 Therapeutic Goals after Spinal Cord Injury
Therapeutic Goals after Spinal Cord Injury
Therapeutic targets or clinical goals | Timeline for human application after SCI | Selected underlying biological mechanisms |
Protection | Within first few weeks | Edema Secondary cell death Inflammation Immune system responses |
Repair (endogenous) | Weeks to months | Angiogenesis Myelination Astrocyte responses |
Regeneration (exogenous) | Weeks to years | Cell transplants Axonal outgrowth Biocompatible substrates |
Recovery | Weeks to years | Activity-dependent training Axonal sprouting Synaptic plasticity Neuronal circuit rewiring |
Repair includes endogenous cellular and tissue responses that are spontaneously activated after injury or need appropriate stimulation to provide a benefit. For example, stimulation of appropriate angiogenesis or decreasing widespread demyelination is likely to be beneficial.3,4 Current thinking also suggests astrogliosis after SCI is maladaptive to functional recovery, and limiting such responses by astrocytes may be a worthy therapeutic target.5–7 Interventions that enhance the expression of required growth factors, stimulate the proliferation and differentiation of resident neural progenitors, or activate endogenous “developmental programs” to facilitate intrinsic neuronal outgrowth are also areas of active preclinical investigation.1,8
Regeneration is often used as a synonym for repair, but here the emphasis is on exogenous interventions that specifically stimulate or facilitate outgrowth from severed axons, as well as strategies, such as cell and biocompatible material transplants, that replace lost tissue or provide scaffolds for neural growth.9 Obvious approaches include transplantation of autologous or allograft neural stem cells or neural progenitors.10 Although cell transplantation is a most promising therapeutic intervention, at this time there is little scientific consensus as to what are the most appropriate cellular candidates for transplantation after SCI. In addition, different therapeutic goals may require different cell transplant phenotypes. Further preclinical studies are essential before this approach will have a meaningful and widespread clinical application.
Recovery of a clinically meaningful functional capacity is the ultimate goal and may necessarily involve all the above approaches, but activity-dependent rehabilitation alone can provide benefit. The underlying basis is collectively referred to as neuroplasticity, which includes a variety of mechanisms, such as the formation of novel synaptic connections by axonal sprouts from existing (i.e., preserved) fibers, to alterations in synaptic strength, or the rewiring of functional circuits within intact (uninjured) spinal and brain regions.11 Treatments, such as bodyweight-supported walking, robot-assisted training, and functional electric stimulation, capitalize on and facilitate plasticity.12,13 Activity-dependent efforts are also important to consolidate any functional anatomical repair induced by a biological intervention that protects, repairs, or regenerates the injured cord.
SCI is a complex disorder, and functional recovery will undoubtedly require more than one type of intervention. Many pre-clinical investigations are examining the combined or sequential manipulations of promoters, inhibitors, intracellular modulators of axonal growth, cell transplants, and activity-dependent training.14,15 The steps for the clinical translation of combinatorial therapies are necessarily more involved because each individual component, as well as the proposed combination, must be examined for safety at both the preclinical and the clinical level before any evaluation for efficacy can begin.
 Preclinical Translation of Discoveries
Preclinical Translation of Discoveries
A disconnect between preclinical and clinical results is undesirable, especially considering the extensive investment of time and money in a translation process. Thus, the adoption of high-quality preclinical protocols, with blinded assessments, adequate power, and the incorporation of “functional” outcome measures similar to those used in a human study, provide increased confidence. Likewise, most scientists would agree that the optimal pre-clinical translation process would include independent replication of promising pre-clinical strategies.16 Furthermore, in the case of SCI, independent replication efforts might involve the use of different SCI models (e.g., severe vs moderate injury, lacerating vs contusive SCI) or variations of the treatment paradigms. This would establish both the relevance and the robustness of the initial discovery. In addition, finding similar beneficial outcomes of the experimental intervention after SCI in different species would demonstrate the fundamental nature of the therapeutic target and increase the likelihood that the treatment would benefit humans. Finally, if a therapy purports to be a useful acute treatment, it should be demonstrated to have benefit in animals when administered within a clinically relevant time frame (in most circumstances, at least several hours after the initial spinal injury). If an intervention is suggested to benefit people living with chronic SCI, then it should be shown to be effective in a chronic animal model.
As outlined in Table 30.2, there are several characteristics of an experimental treatment that should be established prior to beginning a human study. Nevertheless, some experimental interventions enter into clinical trials without being directly studied in a preclinical animal model of the human disorder. This may occur, for example, if the treatment has a previous clinical use for a different disorder but involves a related clinical therapeutic target. The advantages for such a translational path include a prior understanding of the safety and toxicology of the treatment in humans. Thus, when translated to SCI, there is reduced risk that the intervention might deteriorate neurological function or have other adverse effects (Fig. 30.1).
 Spinal Cord Injury Clinical Trial Process
Spinal Cord Injury Clinical Trial Process
Initial guidelines for the conduct of valid SCI clinical trials have become progressively more important. The development of basic cell culture and surgical transplant technologies is relatively inexpensive and requires only modest biological or surgical expertise. Thus there has been a rapid expansion of “for-profit” clinics offering such treatments. The increasing attractiveness of these transplantation practices has understandable appeal to patients when presented as a “cure,” but patients’ desperation can be exploited. The claims of beneficial outcomes are often based on media reports that are powerfully emotive, but unsubstantiated, and usually rely on anecdotal testimonials from hopeful patients or biased practitioners who stand to profit.
Table 30.2 Necessary Characterization and Development of an Experimental Treatment Prior to Human Study
Therapeutic trait | Approach | Outcome |
Temporal “window of opportunity” | Test different time limits for therapeutic strategy after SCI | Determine early and late time points for the therapeutic benefit |
Formulation | Examine different forms of drug, types of cells, or rehabilitation training programs | Identify which formulation provides best actions with minimal adverse effects |
Route of administration | Investigate the most effective route to achieve therapeutic benefit (e.g., intravenous, intrathecal, intraparenchymal) | Determine best potential route for administering intervention in human patients |
Dosage | Test different levels (dose response) of therapy (e.g., drug doses, cell numbers, or length and number of rehabilitation sessions) (also see adverse effects) | Define the optimal dosage that will potentially achieve a meaningful benefit, without unwanted side effects or adverse events |
Adverse effects | Determine doses that cause unwanted side effects or adverse events | Identify tolerable and maximal dosages of therapeutic intervention |
Fate of drug, transplanted cells, or rehabilitation strategy | Drugs: pharmacodynamics (action of drug on body) and pharmacokinetics (actions of body on drug—absorption, diffusion, metabolism, excretion) Cells: integration with host tissue, distribution, survival, tumorigenicity Rehabilitation: discover persistence of rehabilitative benefit | Outline the expected duration (length) of benefit that may be achieved through the therapy Gain better understanding of how the body interacts with the therapeutic intervention |
Mechanism of action | Identify target or biological action of the intervention (e.g., altered biochemical pathway) | Provides information for development of subsequent (next-generation) therapeutics |
Note: Rehabilitation strategies (activity-dependent training programs) should undergo similar characterization.
To ensure public safety, most countries in the developed world have regulatory agencies that have established criteria for an experimental therapy to enter a clinical trial program. Unfortunately, regulatory requirements are only as good as their enforcement, and this has not been uniform across the globe. To provide some objective assistance to a complex series of decisions weighing the possible risks and benefits for a human study, an initial set of SCI clinical trial guidelines was recently developed and published by an international panel of scientists and clinicians. This series of papers detailed the degree of spontaneous recovery after SCI,17 outlined approaches for trial outcome measures,18 discussed inclusion/exclusion criteria and ethics,19 and outlined various trial designs and protocols.20 In addition, the same authors created a document written for the general public and allied health care professionals (www.icord.org).
Each phase of a clinical trial program has distinct goals and thus different parameters, protocols, outcome measures, and end points that can govern the conduct for each stage of investigation. The overall translational path for an experimental intervention through the various stages of preclinical and clinical study might be summarized as shown in Fig. 30.1.
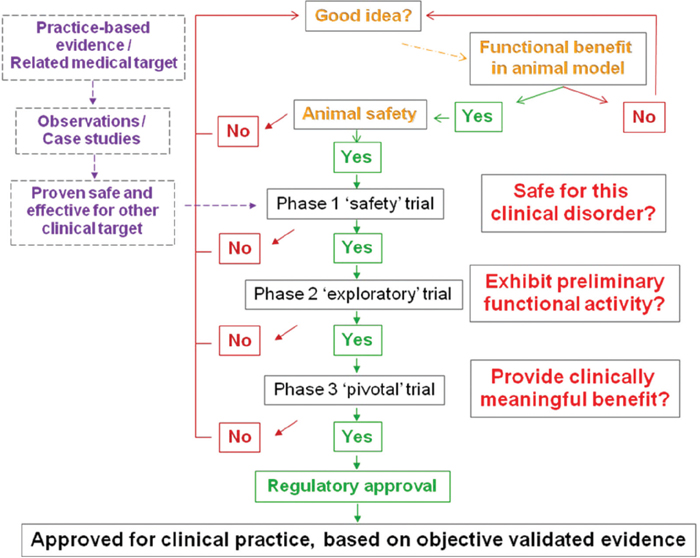
Fig. 30.1 Summary chart of a translational path for a therapeutic intervention from discovery to approval for clinical use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


