CHAPTER 15 Complications of Supracondylar Fractures of the Elbow
NEUROVASCULAR PROBLEMS ASSOCIATED WITH SUPRACONDYLAR FRACTURES
ANATOMY
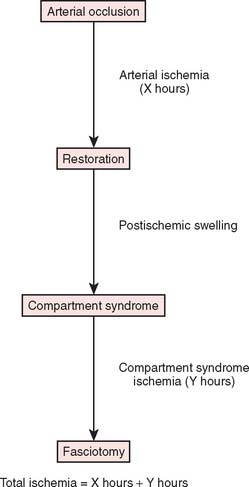
Anterior to the supracondylar area of the distal humerus is the median nerve (Fig. 15-1). In the proximal forearm, the anterior interosseous branch separates to innervate the flexor profundus to the index finger and the flexor pollicis longus and then terminates with the innervation of the pronator quadratus. There is no sensory branch for this nerve. The remainder of the median nerve traverses the forearm and supplies the sensation to the palmar aspect of the thumb, the index finger, the long finger, and the radial aspect of the ring finger.
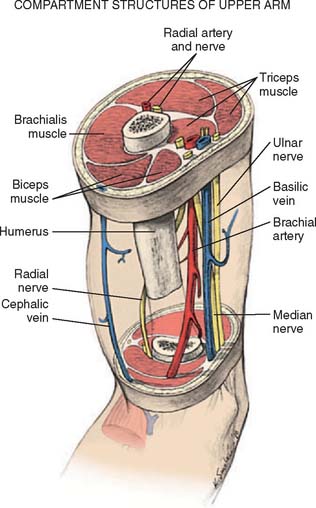
FIGURE 15-1 Major neurovascular structures of the elbow.
(From Mubarak, S. J., and Hargens, A. R.: Compartment Syndromes and Volkmann’s Contracture. Philadelphia, W. B. Saunders, 1981, p. 24.)
The radial nerve lies posterolateral to the usual location of supracondylar fractures and, thus, is less commonly involved (see Fig. 15-1). The ulnar nerve with its posterior location is uncommonly involved with a typical extension-type supracondylar fracture.
The forearm consists of two basic compartments: volar and dorsal (Fig. 15-2). The volar compartment includes the flexors and pronators of the forearm and wrist, which may be further divided into superficial and deep muscle groups. The superficial muscles include the flexor carpi ulnaris, the palmaris longus, the flexor carpi radialis, and the pronator teres. The deeper group of muscles consists of the flexor digitorum superficialis and profundus, the flexor pollicis longus, and the pronator quadratus.
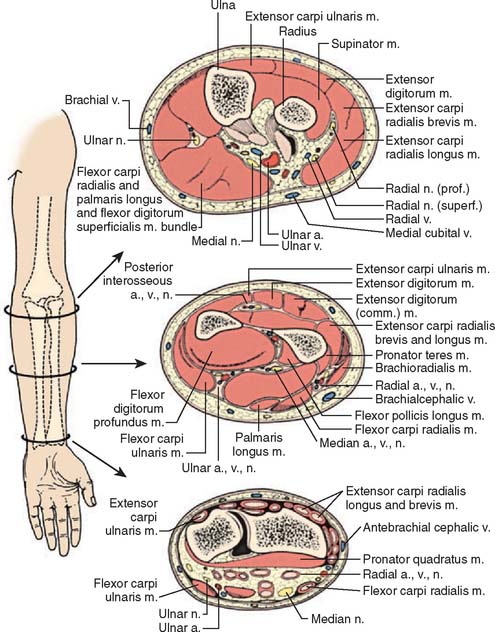
FIGURE 15-2 Forearm compartments: transverse sections through the left forearm at various levels.
(From Mubarak, S. J., and Hargens, A. R.: Compartment Syndromes and Volkmann’s Contracture. Philadelphia, W. B. Saunders, 1981, p. 28.)
ETIOLOGY: NERVE INJURY
Most nerve injuries are associated with type III displaced supracondylar fractures. In a recent study by Louahem et al,46 the most commonly injured nerve was the anterior interosseous branch of the median nerve. This is likely due to its anatomic arrangement of the exclusively motor posterior fascicles which are exposed to the zone of injury, and its tight tethering to the proximal forearm musculature. The second-most commonly involved nerve was the ulnar, followed by the radial nerve. Ulnar nerve injury was most commonly associated with posterolateral fracture patterns due to direct contusion and stretching of the nerve from the medially displaced proximal humeral fragment or edema within the cubital tunnel. Radial nerve injury was consistently associated with posteromedial fractures due to contusion and stretching from the laterally displaced proximal humeral fragment.
Ulnar nerve injury also occurred iatrogenically in 5% of patients during medial percutaneous pin placement in a recent large series.69 The causes of iatrogenic ulnar nerve injury include (1) direct penetration of the nerve or its sheath by the medial pin; (2) constriction of the cubital tunnel by the pin while the elbow is in flexion; (3) medial pin injury to an unstable ulnar nerve, which subluxates or dislocates anteriorly when the elbow is in flexion; and (4) nerve contusion and edema.63 In 2001, Skaggs et al reported on 345 extension-type supracondylar humerus fractures in children treated with closed reduction and percutaneous pin fixation. The use of a medial pin was associated with an iatrogenic ulnar nerve injury in 15% of patients in which the pin was placed with the elbow positioned in hyperflexion. Only 4% of patients sustained nerve injury when the medial pin was placed without hyperflexion, and no iatrogenic injuries occurred in patients treated with all lateral entry pin fixation.69 A displaced supracondylar fracture presenting with an absent radial pulse has a 50% to 60% incidence of associated nerve injury at fracture presentation.19
TREATMENT
After reduction of the fracture and stabilization with percutaneous pinning, re-evaluation of the neurovascular examination is mandatory. On rare occasions, the compromised nerve may recover before the patient’s discharge, but in most incidents, the neurapraxia requires observation and will gradually return over the ensuing months. If after 4 to 6 months, no return of function is noted, electromyelographic and nerve conduction studies to evaluate the status of recovery are recommended. Only rarely have cases been reported of permanent nerve deficits requiring later neurolysis, grafting, or tendon transfer. Nearly all nerves will return to normal function within the first 6 months following the injury.19
Advances in surgical techniques with lateral pin entry fixation have demonstrated significant decreases in iatrogenic ulnar nerve injury and satisfactory mechanical stability in Gartland type II, III and IV fractures.43,69,70 Authors recommend two-pin lateral-entry fixation as the primary mode of percutaneous fixation in all unstable supracondylar humerus fractures with the addition of a third lateral-entry pin or medial pin as needed to achieve fracture stability.
ISCHEMIC INJURIES
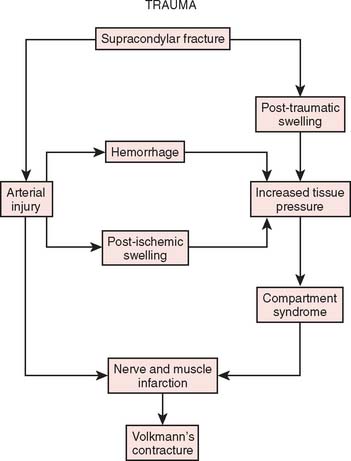
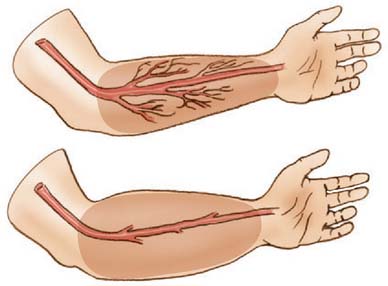
Two basic pathologic processes may result from supracondylar fractures or other injuries to the elbow region that can lead to forearm ischemia: (1) arterial injury and (2) compartment syndrome from hemorrhage or postischemic swelling (Fig. 15-3). An arterial injury may result from laceration, thrombus, embolus, intimal tear, or pseudoaneurysm (Fig. 15-4). Such an injury may cause nerve and muscle ischemia directly or may result in postischemic swelling or hemorrhage, thereby causing a compartment syndrome.
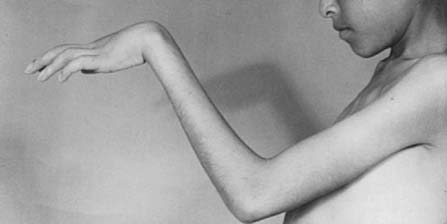
To prevent permanent loss of nerve and muscle function, this condition must be diagnosed promptly and treated correctly. Volkmann’s contracture is the popular term that refers to the end stage of an ischemic injury to the muscles and nerves of the limb (Fig. 15-5). Untreated compartment syndromes and arterial injuries are the primary causes of Volkmann’s contracture. The term Volkmann’s ischemia is nonspecific and should not be used.
ETIOLOGY: ISCHEMIA
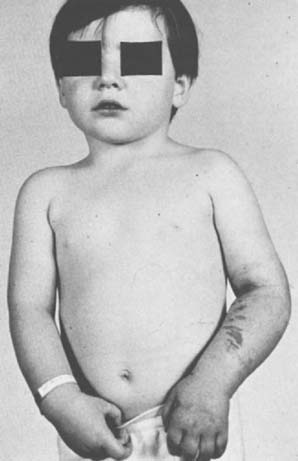
In general, the most common traumatic event that produces a compartment syndrome or an arterial injury about the elbow is the supracondylar fracture of the distal humerus (Fig. 15-6). In 1956, Lipscomb noted that supracondylar fractures were the cause of 48% of Volkmann’s contractures in 92 cases from the Mayo Clinic.45 In 1967, Ehrlich and Lipscomb, in a review of 32 more cases of Volkmann’s contracture, reported that 34% were due to supracondylar fractures and 22% were due to forearm fractures.13 In 1979, Mubarak and Carroll, reporting on 58 Volkmann’s contractures in children (Fig. 15-7), found that supracondylar fractures had caused only 16% of these contractures.56 In most recent studies, compartment syndromes are extremely unusual because of the advent of early closed reduction and percutaneous pinning.
An arterial injury can produce nerve and muscle ischemia directly or the additional problem of a compartment syndrome by one of two mechanisms (see Fig. 15-3).57 First, if the major vessel is lacerated, hemorrhage into the compartment may produce the syndrome. Second, a compartment syndrome may result from postischemic swelling if there is inadequate collateral circulation or if the vessel is only partially occluded, for example, from an arterial spasm or an intimal tear. In this situation, the decreased perfusion and ischemia of both capillaries and muscles will cause an increase in the permeability of the capillary walls. The resulting edema will then cause more ischemia, and a vicious circle may ensue. When there is complete arterial occlusion, a compartment syndrome may develop from postischemic swelling or reperfusion injury after the circulation is restored (Fig. 15-8). When complete arterial occlusion is secondary to massive emboli or prolonged use of a tourniquet in which the circulation is not restored, gangrene rather than compartment syndrome will likely result.
CLINICAL DIAGNOSIS
There is an association between supracondylar fractures, an absent radial pulse, and Volkmann’s contracture. When the concepts of compartment syndrome as a cause for Volkmann’s contracture became popular, forearm fasciotomies became the accepted treatment method to prevent this devastating complication. An absent radial pulse, which is most commonly associated with arterial injury, began to merge with the notion of compartment syndrome. This misconception has no doubt caused many physicians to delay treatment for a compartment syndrome while waiting for the radial pulse to disappear. Owing to these misconceptions, the signs and symptoms of arterial injury compared with those of compartment syndrome will be discussed in detail.
Signs of Compartment Syndrome
Except in the presence of major arterial injury or disease, peripheral pulses and capillary filling are routinely intact in compartment syndrome patients. Although intracompartmental pressures may become high enough to cause ischemia of the muscle and nerve by occluding the microcirculation within the compartment, the pressures are rarely high enough to occlude the major arteries (Fig. 15-9). In our experience, the intracompartmental pressures usually do not exceed 80 mm Hg and are more commonly between 40 and 60 mm Hg. It has been suggested that absent pulses may result from vascular spasm secondary to elevated intracompartmental pressures.12 Mubarak and colleagues have demonstrated that pressurization to as high as 80 mm Hg of the entire anterolateral compartment in a number of dogs produced only occasional transient spasm of the midsize vessels on angiography.
DIFFERENTIAL DIAGNOSIS
Many traumatic events that precipitate a compartment syndrome or arterial injury can also produce a painful, swollen extremity. The diagnosis of the underlying problem (e.g., fracture or contusion) is obvious; the diagnosis of a superimposed ischemia is more difficult. Pain out of proportion to that expected for the injury and any sensory deficit must be explained. A compartment syndrome or an arterial injury also must be differentiated from a nerve injury, which is usually a neurapraxia when it is associated with a closed elbow fracture or dislocation. The clinical findings of these three entities overlap, frequently making the diagnosis difficult, if not impossible, by clinical means. All of these problems may be associated with motor or sensory deficits and pain. Careful clinical evaluation is necessary to differentiate these entities (Table 15-1). As noted earlier, an arterial injury usually results in absent pulses, poor skin color, and decreased skin temperature. In contrast, a compartment syndrome routinely presents with intact peripheral circulation unless the underlying etiology is an arterial injury. A diagnosis of nerve injury is usually made by exclusion of the other two entities. Doppler blood flow studies, arteriography, and pressure measurements are frequently required to aid in the differential diagnosis of these three entities, especially if these problems are present in combination.
TABLE 15-1 Typical Clinical Findings of Compartment Syndrome, Arterial Occlusion, and Neurapraxia
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
From Mubarak, S. J., and Carroll, N. C.: Volkmann’s contracture in children: aetiology and prevention. J. Bone Surg. 61B:290, 1979.
Differentiation of these entities is important because therapy for each is radically different. The neurapraxia accompanying a closed fracture is usually best treated by observation. Arterial injuries warrant immediate operative repair of the vessel, and a compartment syndrome necessitates immediate decompressive fasciotomy.
TREATMENT
Arterial Injury
When an arterial injury associated with a supracondylar fracture is suspected, a Doppler examination should be performed. The velocity Doppler is an integral instrument in assessing the presence of peripheral pulses and is very useful for noninvasive documentation of pulses in the presence of a markedly swollen extremity. A quantitative Doppler technique has been described by Schoenecker and colleagues66 to detect significant asymmetry between the injured and an uninjured extremity in children with type III supracondylar humerus fractures. Arteriography is not recommended in an acute situation.67 Shaw and associates noted the risk of arteriography to be the following: (1) prolongation of ischemic time between fracture and reduction; (2) arterial damage at the catheter insertion site; and (3) allergy to contrast material.67
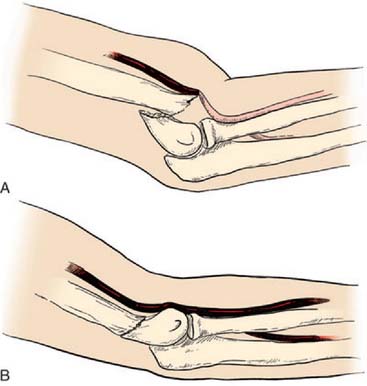
After confirmation of distal forearm ischemia, an attempt to better align the fracture fragments should be made immediately in the emergency room. In extension-type fractures, this is accomplished by extending the elbow, correcting any coronal plane deformity, and reducing the fracture by bringing the proximal fragment posteriorly and the distal fragment anteriorly (Fig. 15-10). Often, this simple maneuver will immediately restore distal circulation.33 If the distal circulation is not restored, a vascular surgeon should be notified, and the patient should be taken immediately to the operating room. All authors agree that the fracture should be reduced and stabilized by percutaneous pinning or, if necessary, open reduction and fixation. If the radial pulse does not return within 30 minutes, and signs of forearm and hand ischemia continue to be evident, then exploration of the brachial artery at the fracture site is recommended. In these circumstances, prophylactic fasciotomy of the forearm should be considered after brachial artery repair if the period of ischemia is more than 4 hours. An algorithm for the treatment of supracondylar humerus fractures associated with forearm and hand ischemia is represented in Figure 15-11.
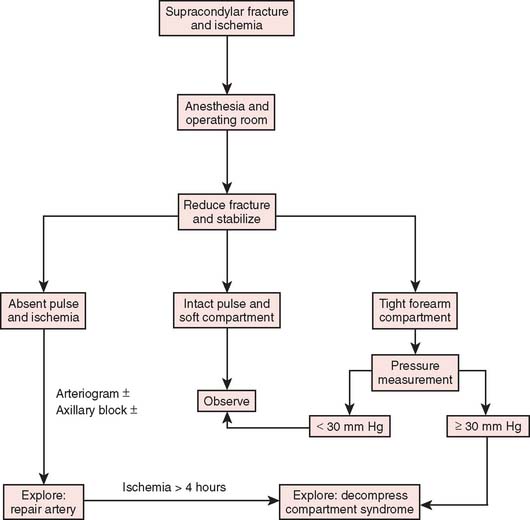
FIGURE 15-11 Scheme for management of supracondylar fractures associated with upper extremity ischemia.
(From Mubarak, S. J., and Hargens, A. R.: Compartment Syndromes and Volkmann’s Contracture. Philadelphia, W. B. Saunders, 1981, p. 144.)
Shaw and colleagues67 explored three cases and documented intimal tears with thrombus obstructing the brachial artery lumen. In two patients, the injured segment was excised and replaced by a saphenous vein graft; and prophylactic fasciotomy was also performed. One patient was noted to have brachial artery entrapment at the fracture site that was appropriately released.
Schoenecker and associates66 recommend brachial artery exploration if Doppler-detectable pulses did not return within 30 minutes after fracture reduction. A vascular surgeon assisted with the exploration. Three of seven patients demonstrated interluminal damage or transsection, requiring saphenous vein graft. Four others demonstrated kinking or entrapment of the artery at the fracture site, with re-establishment of the pulses after mobilization. Garbuz and coworkers19 explored five brachial arteries and found a similar ratio of luminal damage, laceration, and entrapment of the arteries. One patient was treated with ligation, and had long-term claudication symptoms. Eight of 11 patients who initially had an absent radial pulse demonstrated a return of the pulse after the closed reduction. In three children, the radial pulse did not return, but no further treatment was required because the forearm and hand remained pink without any further neurologic deficits. This is known in the orthopedic literature as the “pulseless pink hand.”
The Vancouver study group reviewed the pulseless pink hand in 13 patients following closed reduction and percutaneous pinning of the supracondylar fracture.65 They recommended color flow duplex scanning and MRA as a noninvasive safe technique for evaluating brachial artery patency and collateral circulation around the elbow. The vascular injuries in the 13 patients were studied; four had a thrombus or intimal tear. These patients underwent vein patch graft angioplasty. Urokinase infusion was used intra-arterially in four patients, and open thrombectomy was used in one. At follow-up, MRA studies of these patients showed a high rate of asymptomatic reocclusion and residual stenosis of the brachial artery. Thus, the investigators called into question the need for vascular reconstruction of intimal tears when the patient has a pink, well-perfused hand and MRA or other noninvasive studies that demonstrate adequate collateral circulation. They strongly recommended observation as the mainstay of treatment for these patients.
In a recent survey of pediatric orthopedic surgeons, 60.5% of responding surgeons would monitor a pulseless pink hand for at least 24 hours following reduction and pinning. If the hand remained pulseless after 24 hours, the majority of respondents (61.2%) would continue to observe the extremity and monitor for adequate collateral circulation.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree