Chapter 32 Complications of Catheter Ablation of Cardiac Arrhythmias
Reported rates of major complications following contemporary catheter ablation procedures vary by as much as five- to eightfold between various types of ablation procedures, ranging from 0.8% for supraventricular tachycardia (SVT), 3.4% for idiopathic ventricular tachycardia (VT), 5.2% for atrial fibrillation (AF), and 6.0% for VT associated with structural heart disease. Death is a rare complication of catheter ablation, occurring in 0.11% to 0.30% of patients with regular SVT, and in 0.31% of those with VT. Transseptal catheterization appears to be the cause of death in 0.2% of procedures.1
Local Vascular Complications
Complications at the catheter insertion sites are among the most common problems observed following catheter ablation procedures, estimated to occur in about 2% to 6% of procedures, and can cause significant morbidity. Local vascular complications are higher among females, older adults, obese patients, and those with preexisting peripheral vascular disease. Additionally, the risk is related to the type of procedure performed (right or left heart catheterization), the size and number of sheaths and catheters used during the procedure, as well as the associated periprocedural use of anticoagulant or antiplatelet agents.2 The rates of complications related to the site of vascular access are higher following catheter ablation of AF (1.8%) and VT in patients with structural heart disease (0.7% to 4.7%) compared with ablation of SVT (0.4%).1
Bleeding is the most common vascular complication. This can simply result in a local hematoma of little clinical significance. Vascular laceration can cause large hematomas in the groin and thigh. Acute bleeding generally can be controlled with prolonged manual compression. Groin hematomas usually resolve in 1 to 2 weeks if ongoing bleeding is stopped. Large hematomas can require blood transfusion, but surgical repair is rarely required. Arteriovenous fistulas and pseudoaneurysms need to be excluded in the setting of large or continually expanding hematomas. Retroperitoneal hematomas are often the result of arterial puncture above the inguinal ligament, allowing bleeding and hematoma to extend to the retroperitoneal space. Retroperitoneal hematomas should be suspected in the setting of a marked drop in hematocrit or unexplained hypotension or flank pain. Abdominal computed tomography (CT) scanning or ultrasound is required to confirm the diagnosis. Retroperitoneal bleeds are generally managed conservatively (bed rest, blood transfusion). Catheter or surgical interventions, however, can occasionally be required.3
Femoral arteriovenous fistulas commonly result when bleeding from the arterial puncture tracks into the adjacent venous puncture. Arteriovenous fistulas are more likely to arise when arterial and venous punctures are performed on the same side or when arterial puncture is performed below the common femoral artery, where several superficial branches of the femoral vein overlie the femoral artery. Diagnosis is made on examination with the finding of a pulsatile mass with a continuous “to and fro” bruit and confirmed by ultrasound. Many of the iatrogenic arteriovenous fistulas are small and close spontaneously within 1 year, but ultrasound-guided compression or surgical repair can be necessary. Because cardiac volume overload and limb damage are highly unlikely with persistent arteriovenous fistulas, conservative management for at least 1 year is reasonable.2
Cardiac Perforation
Incidence
Previous studies reported the incidence of cardiac perforation with catheter-based interventions as follows: 0.8% for all procedures, 1.5% to 4.7% for valvuloplasty, 0.5% to 0.8% for angioplasty-atherectomy, 0.01% for diagnostic catheterization, 0.1% to 0.2% for electrophysiological (EP) studies, 0.2% for SVT ablation, 0.4% to 2.7% for VT ablation, and 0.5% to 4.0% for left atrial (LA) ablation.1,4,5
The incidence of procedure-related cardiac tamponade increases with the increasing number of catheter ablation procedures for the treatment of AF, which typically involves one or more atrial septal punctures, extensive intracardiac catheter manipulation and ablation within the thin-walled LA, and the need for high levels of systemic anticoagulation during the procedure.2,6
Mechanism
Cardiac perforation can be caused by mechanical trauma secondary to catheter manipulation, myocardial tissue rupture due to radiofrequency (RF) ablation, or inadvertent injury during atrial septal puncture. Extensive catheter manipulation, especially in areas with thin walls such as the LA roof, right ventricular (RV) free wall, RV outflow tract (RVOT), RV apex, and atrial appendages, can cause mechanical tear of the chamber wall. Cardiac perforation can also occur away from the area of mapping and ablation, usually caused by penetration of an unattended RV catheter in the setting of rapid and vigorous heart action because of high-dose isoproterenol infusion. Inadvertent puncture of the right atrial (RA) free wall or lateral LA wall can occur during transseptal catheterization. High RF power output, especially when using large- or irrigated-tip ablation catheters, can increase the risk of cardiac perforation. An audible pop associated with an abrupt rise in impedance is heard in many patients who develop tamponade. Popping occurs because of tissue boiling causing myocardial rupture, and is increased by irrigated-tip ablation, high tissue-catheter interface flow, poor or unstable tissue contact, and high catheter tip temperature.2,6
Detection
Prompt recognition and management of cardiac perforation are critical to prevent the development of cardiac tamponade and potentially life-threatening hemodynamic collapse. Although the presentation often is dramatic with abrupt hypotension, it can be insidious with a more gradual fall in blood pressure. The use of an arterial line that provides continuous blood pressure monitoring can help detect early hemodynamic compromise. Sinus tachycardia is common in the setting of cardiac tamponade that gradually develops. Nonetheless, the absence of tachycardia does not exclude cardiac tamponade; in fact sinus bradycardia can develop secondary to a vagal reflex in the setting of a rapidly developing cardiac tamponade. Any chest pain that persists beyond the completion of an ablation lesion, especially if associated with hypotension and diaphoresis, should alert the operator to the possible development of pericardial effusion.2,6–8
Assessment of the cardiac silhouette fluoroscopically can provide the first clue, especially if a similar assessment was made at baseline. Decreased excursion of the lateral heart border on fluoroscopy in the left anterior oblique (LAO) projection, indicating accumulating pericardial effusion, usually can be seen well before a drop in blood pressure and prior to progression to cardiac tamponade. Some operators obtain a baseline LAO cine image at the onset of a procedure to serve as a reference for comparison during the procedure, followed by intermittent evaluation of the same fluoroscopic projection during the procedure.2,6–8
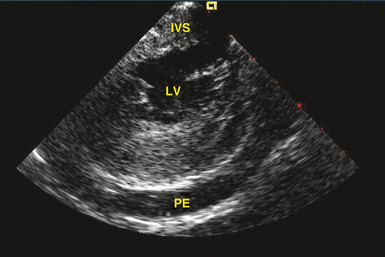
Transthoracic echocardiography is the most definitive method for confirming the development of pericardial effusion. Intracardiac echocardiography (ICE), commonly used to guide transseptal puncture for LA procedures, also can facilitate the early detection of pericardial effusions before the emergence of tamponade physiology. Most of the pericardial space is not visualized from the RA imaging venue used to visualize the interatrial septum, and limited catheter rotation is usually required. Advancing the ICE catheter into the RV and rotating the transducer against the interventricular septum can readily identify pericardial effusions (Fig. 32-1).
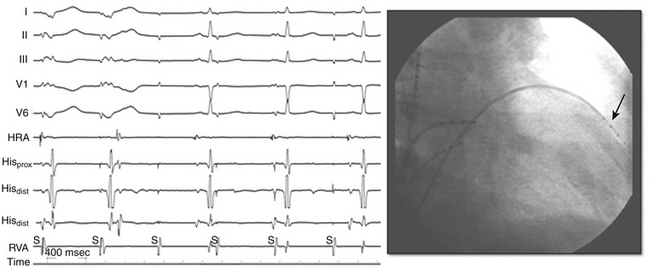
Perforation without tamponade may occur during placement of diagnostic catheters or while accessing the LA by transseptal catheterization; detection at this time can prevent progression to tamponade but requires a high index of suspicion. Several clues should alert the operator to the possibility of cardiac perforation, including a ventricular catheter that reaches the edge of the cardiac silhouette, high pacing thresholds at sites despite normal electrograms and apparent good tissue contact on fluoroscopy, a right bundle branch block (RBBB) complex during “RV” pacing, and a catheter tip position discordant from where it should be (i.e., an RV catheter too far leftward with intended RV apical location; Fig. 32-2). With atrial septal puncture for accessing the left heart chambers, the aorta can be inadvertently entered; if only the needle enters the aorta and this is recognized before advancing the dilator and sheath, the needle can be withdrawn and the patient monitored for stability of vital signs and with echocardiography. The procedure can be continued if there is no accumulation of pericardial fluid after 15 to 30 minutes of monitoring. If the dilator and sheath have been advanced into the aortic root before the error is recognized, it is imperative that the sheath not be removed immediately, as this can result in immediate and perhaps irretrievable hemodynamic collapse due to intractable bleeding into the pericardial space.
Management
The management of pericardial effusion is largely determined by its relative size and hemodynamic effect. Trivial pericardial effusions, if recognized early during the procedure, should be monitored continuously but do not warrant termination of the procedure. For larger effusions, the procedure should be terminated and anticoagulation, if administered, should be reversed. Protamine is used to reverse the effects of heparin, and activated factor VII, fresh frozen plasma, and vitamin K can be used in patients with therapeutic anticoagulation with warfarin. Intravenous fluids, vasopressors, and transfusion of blood products can be required, depending on the extent of the effusion and the severity of hemodynamic decompensation. Intravenous atropine can be of value in patients with increased vagal tone.3,9
Although pericardiocentesis typically is required for large pericardial effusions, smaller effusions manifesting signs of cardiac tamponade, or both, and can effectively restore hemodynamic function, it carries the potential risk of cardiac chamber laceration, inadvertent puncture of the RV, pneumothorax, and infection. In a subset of patients (especially older female patients with a small to moderate amount of pericardial effusion who are not anticoagulated), a conservative approach incorporating administration of intravenous fluids and vasopressors to address the hemodynamic consequences of cardiac tamponade was found in a recent report a reasonable initial strategy and could obviate the need for emergency pericardiocentesis.2,7
Pericardiocentesis should be performed promptly when indicated, because there is usually a narrow therapeutic time window for intervention before critical hemodynamic compromise ensues. The procedure can be performed under fluoroscopic guidance (Video 22)  . Echocardiography can also be used to guide pericardiocentesis (Video 23
. Echocardiography can also be used to guide pericardiocentesis (Video 23  ). When echocardiography is not readily available, fluoroscopy is usually adequate to guide a safe procedure, and reliance on transthoracic echocardiography should not lead to unnecessary delay in diagnosis or therapy. The technique of percutaneous pericardiocentesis is discussed in Chapter 27. In most patients, an indwelling catheter is required for a short interval after initial drainage to confirm that the bleeding has stopped and that no effusion is reaccumulating. In the event of persistence or rapid reaccumulation of the effusion, exploration by thoracic surgery can be required. Up to 13.3% of LA perforations during AF ablation require surgical closure. Autotransfusion of blood removed from the pericardial space can be of value in patients with persistent bleeding, and is best done using an autologous blood recovery system, because direct autotransfusion can result in a systemic inflammatory response.3,6,9
). When echocardiography is not readily available, fluoroscopy is usually adequate to guide a safe procedure, and reliance on transthoracic echocardiography should not lead to unnecessary delay in diagnosis or therapy. The technique of percutaneous pericardiocentesis is discussed in Chapter 27. In most patients, an indwelling catheter is required for a short interval after initial drainage to confirm that the bleeding has stopped and that no effusion is reaccumulating. In the event of persistence or rapid reaccumulation of the effusion, exploration by thoracic surgery can be required. Up to 13.3% of LA perforations during AF ablation require surgical closure. Autotransfusion of blood removed from the pericardial space can be of value in patients with persistent bleeding, and is best done using an autologous blood recovery system, because direct autotransfusion can result in a systemic inflammatory response.3,6,9
Early pericarditis after cardiac perforation is common. In one report, 53.3% of such patients had persistent chest pain after effusion evacuation and removal of the pericardial catheter suggestive of pericardial inflammation. Nonsteroidal antiinflammatory agents are adequate in most patients. Additionally, intrapericardial steroids (triamcinolone, 20 mg) can help reduce pericardial inflammation. Subacute reaccumulation of pericardial fluid suggestive of postcardiac injury syndrome or inflammatory pericarditis can also occur, requiring repeat pericardiocentesis.3,9
Thromboembolism
Systemic thromboembolism can complicate EP procedures in the left side of the heart. The rate of thromboembolic events is 0.4% to 2.1% for AF ablation and up to 2.8% for ablation of VT originating in the left ventricle (LV).1,4,10 In patients undergoing AF ablation, history of a prior cerebrovascular event is the most potent individual risk factor for post-ablation cerebrovascular events and is associated with a ninefold increased risk of periprocedural stroke. Additionally, the incidence of periprocedural stroke increases in a step-wise fashion with an increasing CHADS2 score (congestive heart failure, hypertension, age >75 years, diabetes, and previous stroke/transient ischemic attack).10
Mechanism
Potential sources of emboli include thrombus formation on high-profile wires, catheters, and sheaths inserted in the LA or LV, char formation at the tip of the ablation catheter or at the site of ablation, thrombi or air passing through a patent foramen ovale or transseptal puncture, and dislodgment of a preexistent LA or LV thrombus by catheter manipulation. Additionally, new LA appendage thrombi may develop in patients with persistent AF after planned or inadvertent conversion to sinus rhythm, especially in the setting of insufficient levels of anticoagulation before, during, or after ablation. Furthermore, endocardial disruption from the ablation lesions can potentially become a nidus for thrombus formation. Also, aortic atheroembolism can occur during retrograde transaortic LV access for ablation of VTs or atrioventricular (AV) bypass tracts (BTs), and it tends to be associated with difficulty in manipulation of catheters within a severely diseased aorta.10
Thromboembolic events typically occur within 24 hours of the ablation procedure, with the high-risk period extending for the first 2 weeks following ablation. Cerebral thromboembolism is most common, but emboli can also involve the coronary, abdominal, or peripheral vascular circulations. Although silent cerebral thromboembolism has been reported, its incidence and clinical significance are unknown.11
Prevention
Prevention remains the best strategy in minimizing cerebrovascular events during left heart mapping and ablation, and this may be achieved by the following: (1) preprocedural transthoracic or transesophageal echocardiography (TEE) in patients with AF as well as those with ischemic VT with LV dysfunction; (2) aggressive intraprocedural anticoagulation, including early heparin administration (once vascular access is obtained), followed by continuous infusion to maintain the activated clotting time at greater than 300 seconds; (3) meticulous attention to sheath management, including constant infusion of heparinized saline and air filters; (4) minimizing char formation during lesion creation by regulating power delivery to prevent abrupt impedance rise; and (5) using ICE during AF ablation for early detection of intracardiac thrombi and accelerated bubble formation consistent with endocardial tissue disruption with RF application. Open-irrigation RF ablation or cryoablation, compared with standard 4- or 8-mm solid tip or closed irrigation electrodes, can potentially decrease the formation of char and thrombus at the tip of the ablation catheter.10 Administration of large doses of protamine on completion of the ablation procedure to reverse heparin abruptly can potentially promote thrombogenesis and warrants further evaluation to confirm its safety. Continuation of warfarin at a therapeutic level at the time of AF ablation can potentially be a better alternative to strategies that use bridging with heparin or enoxaparin, as it eliminates a period of inadequate anticoagulation immediately following the ablation procedure, a critical period for thromboembolic risk because of the inflammation and irritation inherently associated with ablation.12,13
Air Embolism
Vascular air embolism is a potentially life-threatening event that can occur during left and right heart procedures. Because many cases of venous air embolism go unnoticed, the true incidence of this complication is unknown. Air embolism has been reported in the interventional radiology literature at an incidence of 0.13%.2,3,9
Mechanism
Paradoxical air embolization into the arterial circulation can occur through direct passage of air into the arterial system via anomalous structures such as an atrial or ventricular septal defect, a patent foramen ovale, or pulmonary arterial-venous malformations. Direct arterial air embolism is caused by the introduction of air into the LA via the transseptal sheath as well as via long vascular sheaths occasionally used for catheter stabilization during transaortic mapping and ablation in the LV.3,9
Clinical Presentation
Most cases of venous air embolism are subclinical and do not result in untoward outcomes, and even when symptomatic, they go unrecognized because of the nonspecific nature of clinical presentation that can mimic other cardiac, pulmonary, and neurological dysfunctions. Therefore, a high index of suspicion is necessary to establish the diagnosis.3
The outcome of venous air embolism is directly related to the amount of air and the rate at which it enters the vein. Spontaneously breathing patients can experience more serious consequences than those under controlled positive-pressure ventilation because they generate negative intrathoracic pressure during the respiratory cycle, facilitating air entrainment. Awake patients typically manifest shortness of breath, continuous coughing, chest pain, and a sense of “impending doom.” Jugular venous distention, hypotension, tachycardia, and electrocardiographic (ECG) signs of right heart strain (ST-T wave abnormalities) can be observed. Severe cases are characterized by cardiovascular collapse.3,9
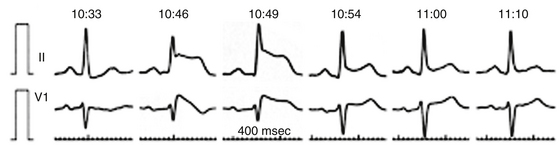
Arterial air emboli can distribute to almost any organ and can have devastating clinical sequelae. Direct cerebral air embolism can be associated with altered mental status, seizures, and focal neurological signs. A common presentation of air embolism during LA mapping and ablation is acute inferior ischemia, heart block, or both (Fig. 32-3). This reflects preferential downstream migration of air emboli into the right coronary artery.
Detection
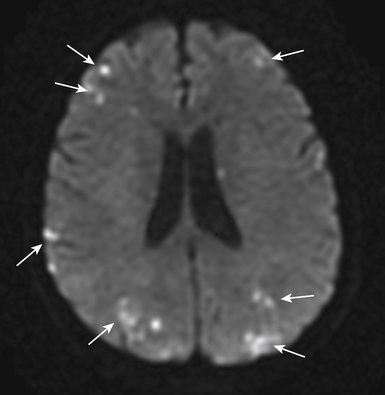
Routine diagnostic modalities to identify air embolism in the terminal arterial circulations lack sensitivity, and diagnosis is typically based on the appropriate clinical scenario, with possible air identified in cardiac chambers. Air in the RV or pulmonary artery can be visualized on fluoroscopy as well as transthoracic echocardiography. TEE is currently the most sensitive monitoring device for venous air embolism. Prompt CT or magnetic resonance (MR) imaging obtained before the intravascular air is absorbed may show multiple serpiginous hypodensities representing air in the cerebral vasculature, with or without acute infarction, but imaging later in the course of the disease shows diffuse acute infarcts (Fig. 32-4).3,9
Prevention
The optimal management of air embolism is prevention. Careful sheath management, including constant infusion of heparinized saline and air filters, should be observed. Although air can be introduced through the infusion line, it can also occur with suction when catheters are removed. Therefore, whenever catheters are removed, they need to be withdrawn slowly to minimize suction effects and the fluid column within the sheath should be aspirated simultaneously. The sheath should then be aspirated and irrigated to ascertain that neither air nor blood has collected in the sheath.3 Importantly, the entire volume of the sheath should be aspirated after initial deployment as well as after each time a catheter is removed and reinserted, in order to ensure that a continuous column of fluid is present in the sheath and disallowing the possibility of trapped air that could otherwise be introduced when a catheter is advanced through the sheath. Testing to ascertain the sheath’s capacity prior to insertion is a good practice.
Management
Initially, it is essential to take all measures necessary to prevent further air embolization. For venous air embolism, placing the patient in the left lateral decubitus and Trendelenburg position helps air remain in the RA, where it will not contribute to an “air lock” in the RVOT. Additionally, direct extraction of air from the venous circulation if localized in the RA or RV can be attempted by aspiration from a central venous or pulmonary artery catheter. Air aspiration should be performed with the patient supine or in a Trendelenburg position while holding his or her breath at the end of inspiration or during a Valsalva maneuver.14
Administration of intravenous fluids and vasopressors can be necessary for hemodynamic support. Supplemental 100% oxygen therapy can reduce the size of the air embolus by increasing the rate of nitrogen absorption from air bubbles. Because gas bubbles are not buoyant enough to counteract arterial blood flow, these measures are more suitable for treating venous and RV air emboli.14
When cerebral air embolism is suspected, it is important to maximize cerebral perfusion by administration of fluids and supplemental oxygen. Hyperbaric oxygen therapy is regarded as the treatment of choice in patients with cerebral air embolism and prompt transfer to a hyperbaric oxygen therapy center should be considered. When started within a few hours, hyperbaric oxygen therapy can potentially compress the existing bubbles, speed resolution of bubbles by establishing a high diffusion gradient, improve oxygenation of ischemic tissues, and reduce endothelial thromboinflammatory injury.3,11,14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree