Fig. 29.1
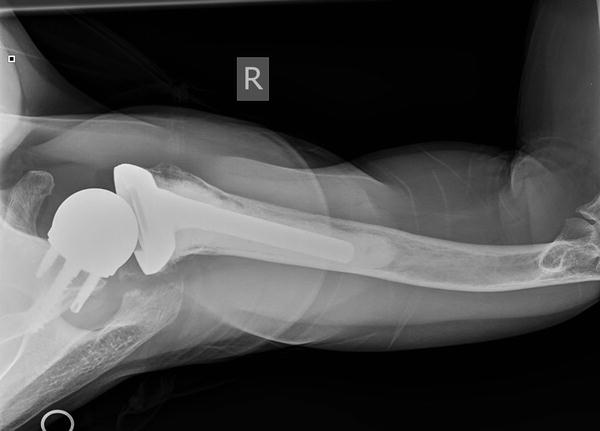
a, b AP and axillary view of a RSA dislocation. c AP view of the same RSA after revision with larger glenosphere and spacers
Scapular Notching
Scapular notching refers to the erosion of the bone inferior along the lateral scapular neck just medial to the glenoid baseplate. It is caused by the impingement and contact made from the inferior medial humeral socket and the scapular neck below the glenoid component. Workup for notching is with a standard anteroposterior (AP) radiograph of the RSA. Scapular notching has been graded 1–4 depending on the size and the depth of the lesion seen on a true AP radiograph of the shoulder (Fig. 29.2) [20]. The full impact of notching has not fully been elucidated, because many reports have detailed a high incidence of notching at medium- to long-term follow-up based on radiographs without much initial clinical consequence.
However, notching may produce a synovitis and inflammation creating pain with loss of motion or osteolysis from particle debris. Severe notching can erode the glenoid bone resulting in a loosened glenoid component necessitating revision or conversion to hemiarthroplasty. Therefore, there is a concern that scapular notching will be a problem for long-term implants. It has been reported to occur in up to 96 % of patients, but depends on certain prosthetic designs, and anatomic and surgical factors [20]. It is a particular concern in prostheses such as the Grammont-style prostheses, but has been reported to be as low of an incidence of 0 % with prostheses with a more lateralized center of rotation, more anatomic neck-shaft angle, and a lowered position of the glenosphere on the glenoid [21].
In a publication with 8–12 years of follow-up on patients with a Grammont-style RSA, they found 88 % of their 60 patients demonstrated scapular notching. Many patients (49 %) had grade 4 notching on radiographs at long-term follow-up which they found was more common utilizing the superolateral approach rather than the deltopectoral approach. Their relatively large incidence of notching overall and high-grade notching present at longer follow-up does suggest that notching may be a progressive phenomenon. Furthermore, although they had a low rate of loosening, there is still concern long term from the notching progressing to loosening and being a major source of late failures [15].
Sadoghi et al. found that scapular notching did not seem to change the clinical outcome of patients until long-term follow-up. For the 12 patients who had follow-up for more than 60 months, the patients who had scapular notching on X-ray had decreased range of motion and increased pain compared to those who had no notching [22]. Others have also found decreased range of motion and increased pain scores in patients with notching [23].
In a cadaveric study on humeral component version using the Aequalis system, Stephenson et al. found that placing the component in 20°–40° of retroversion gave the best combination of motion and decreased impingement against the inferior glenoid rim or inferior scapular neck [24]. It has also been recommended to place the glenosphere in 10°–20° of inferior tilt to reduce notching. Jobin et al. [25] found that patients who had the glenosphere superior tilted had a greater amount of notching at follow-up.
Another paper analyzing scapular notching during medium-term follow-up of their patients who had undergone RSA with a Grammont-style, Delta III system found 44 % of patients developed notching—and in all of them, it was present starting at 14 months. They did not see progression in notching after 14 months indicating to them that notching becomes static after that point. They found that an inferior placed glenosphere (most significant) and implants with a lower glenosphere–scapular neck angle were less likely to develop scapular notching. Furthermore, they did show that the presence of notching correlated to a decreased range of motion in abduction and flexion and worse clinical indicators such as the Constant score and subjective shoulder score [23].
Based on anatomy of known scapulae, de Wilde et al. used a 2D computer system model to analyze factors leading to notching. An increased humeral neck-shaft inclination (from 155° to 145°) decreased notching. A shallower cup depth decreased notching. A lateralization of the center of rotation (from 0 to 5 mm) decreased notching. A downward inclination of 10° compared to 0° decreased notching. An increase in the glenosphere radius from 18 to 21 mm decreased notching. A prosthesis overhang from 0 to 5 mm decreased notching. Glenosphere overhang by inferiorly implanting the baseplate was the most important factor leading to decreased notching potential [26]. Gutierrez et al. [27] found similar results.
A further group compared the incidence of notching in their patient population from one implant design with a neck-shaft angle of 155° and no center of rotation offset to one with a neck-shaft angle of 143° and a 2.5 mm center of rotation lateral offset. The incidence of notching in the former group was 60.7 % compared to the later which was 16.2 %. They were unable to distinguish which aspect, the neck-shaft angle or the lateral offset, contributed most to the changes seen in notching, but the decrease in notching was significant [28]. Another group compared an inferiorly offset glenosphere to a standard offset glenosphere using the Tornier system. They found that the rate of scapular notching was not significantly different between the groups, but the severity of notching was significantly less with an inferiorly offset glenosphere [29].
Individual patient anatomy also seems to play a role in the development of scapular notching. Paisley et al. found that there was a higher incidence of notching in patients with either a short scapular neck or a small scapular neck to glenoid height ratio in their patients who received a Grammont-style arthroplasty. They recommended that with a scapular neck length less than 9 mm on a true AP radiograph, that the surgeon may consider lateralizing the glenosphere by utilizing the BIO-RSA procedure or utilizing a more lateralized prosthesis [30]. Overall, scapular notching could be a problem in the long run and should be avoided. Modern designs are improving to minimize this complication.
Loosening
Despite many technical difficulties in glenoid preparation and glenosphere implantation into often osteoporotic bone, early loosening is now a rare complication. In initial reports, glenoid loosening was a significant concern. However, design improvements have helped correct glenoid fixation. Multiple locking screws, variable angle locking screws, and trabecular metal designs have all been implemented in more recent designs to improve baseplate fixation. Placing the center of rotation at the glenoid has decreased shear stresses across the glenoid. Inferiorly tilting the glenoid has also reduced the shear stresses across the baseplate leading to fewer failures [9]. Therefore, glenoid loosening and baseplate failures are becoming a relatively smaller concern in modern RSAs. Furthermore, glenoid fixation is now so good that it allows for a lateralized center of rotation with its advantages.
Wierks et al. detailed many complications with glenosphere implantation including a fracture, glenoid holes being too large or needing to be redrilled, poor screw purchase, or inability to fit all the screws available in the prosthesis around the glenoid due to glenoid size or inadequate bone stock. Despite these intraoperative “complications,” the one fracture healed and there were no incidences of early glenoid loosening during the follow-up of the 20 patients in their series [2]. The senior author found 2 patients in his group’s series of 137 patients in whom the osteoporotic bone of the glenoid was insufficient to hold the baseplate precluding its use and necessitating a hemiarthroplasty.
As indications expand and RSAs are used more for severe posterior glenoid wear and posterior humeral head subluxation in osteoarthritis (OA) of the shoulder, glenoid fixation is likely to be a concern given the wear already present. In a series of 27 patients who underwent RSA for OA with a Walsh B2 glenoid, results were superior to the senior author compared to conventional arthroplasty [31].
Options for glenoid loosening include conversion to hemiarthroplasty and revision RSA which is very dependent on the residual amount of glenoid bone available for the implantation of the baseplate. Results of conversion to a hemiarthroplasty in one series resulted in continued pain in 3/4 patients [1]. In another series of 6 patients who underwent conversion of failed RSA to hemiarthroplasty, there was residual pain in 5/6 patients with less than 90° of forward flexion. Five of the 6 patients ended up with anterosuperior escape [32].
Bleeding
Hematoma formation after RSA is a concern due to the chance of the hematoma becoming seeded with bacteria leading to infection. In the early literature, 12/58 patients had a clinically significant hematoma [1]. The theory is that the “dead space” created with the RSA from the alteration in the patients’ anatomy allows for blood to pool and create a hematoma. A glenoid with more medialized center of rotation may create more dead space from tensioning the deltoid to a lesser extent than lateralized glenoids. Many surgeons, including the senior author, advocate using surgical drains to decrease hematoma formation. The use of drains and careful attention to hemostasis intraoperatively may be why hematoma is no longer a complication which is even mentioned in many of the papers discussing complications.
Fracture
Periprosthetic humeral stem fractures may occur at the great tuberosity, along the stem, or distal to the stem. Additionally, they may be iatrogenic, intraoperative fractures, or postoperative fractures from a fall or similar trauma. Intraoperative fractures are usually stabilized with hardware once they are appreciated. Postoperative RSA humeral fractures, similar to other humeral fractures and somewhat unlike periprosthetic fractures of the lower extremity, may often be treated nonoperatively. In Melis et al.’s cohort of 60 RSAs with 8- to 12-year follow-up, they had 2 fractures, one at 1 year and one at 9 years’ time. Neither required operative intervention. Alternatively, 2/3 fractures in Groh and Groh’s series of 114 required open reduction internal fixation (ORIF) due to fracture displacement [7] (Fig. 29.3).
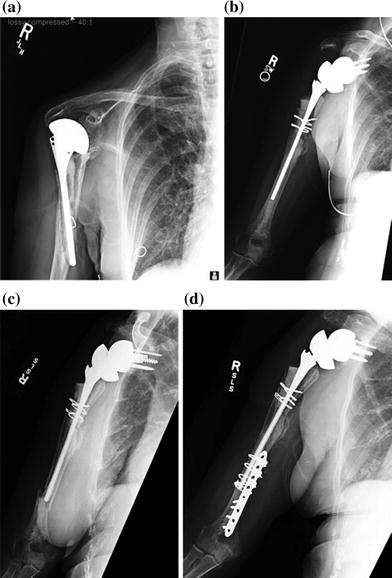

Fig. 29.3
a Failed hemiarthroplasty with periprosthetic fracture. b Conversion to RSA. c Periprosthetic humeral shaft fracture 1.5 years after RSA. d ORIF of the RSA periprosthetic humeral shaft fracture. (Reproduced with permission from Groh and Groh [7])
Intraoperative fractures may occur during reaming or broaching of the humeral calcar or shaft or implantation of the prosthesis. Isolated tuberosity fractures may be held in place with the cement for the stem. As long as the shaft fracture is recognized promptly through visualization, change in resistance of the instrument down the humeral shaft, or change in pitch of the mallet on the broach or implant handle, cerclage wires can be placed around the fracture and distal to hold it reduced while the case resumes. Cerclage wires are used to both reduce the fracture and distal to the fracture to avoid propagation of the fracture. This algorithm has been reported with success in a few series with intraoperative fractures of the humerus [2].
Glenoid fractures that occur intraoperatively may require abandoning the implantation of a RSA in favor for a hemiarthroplasty. However, if just a rim of bone is fractured, and the remainder of the glenoid is intact, baseplate fixation may still be adequate. If so, the fracture may be treated without further intervention. [2].
RSA for Fracture
The use of RSA in proximal humerus fracture situations is becoming increasingly common, particularly in displaced fractures involving the tuberosities of elderly patients with poor bone. Some differences in complications can be seen in RSAs for this indication. The most common complication necessitating further intervention is instability [33]. In comparing the complications of RSA versus hemiarthroplasty for fracture, one series found no difference between the two [34].
The discussion of what to do with the fractured tuberosities is beyond the scope of this chapter. There are many reports of tuberosity problems after the treatment of proximal humeral fractures after RSA such as nonunions, malunions, or resorption. [35, 36] Malunions may lead to bony impingement with resultant notching or instability. Nonunions and tuberosity resorption may lead to instability from the loss of the surrounding bony support. The need for tuberosity fixation may be dependent on design and center of rotation. Most surgeons who use a medialized center of rotation recommend fixation of the tuberosities at least for anterior and posterior security.
Acromion Fracture
Acromion fractures are a recognized complication in RSAs. With rotator cuff arthropathy, the undersurface of the acromion may be eroded from the acetabularization of the acromion from the superior elevation of the humeral head. Furthermore, tensioning the deltoid places increased stresses on the deltoid and ultimately the acromion. The main concern with an acromion fracture is the loss of the structural support for the attachment of the deltoid—the known stabilizer and functional moment arm of the reverse shoulder prosthesis. Therefore, with fracture and possible displacement of the acromion, the deltoid moment arm is shortened leading to functional impairment of the deltoid.
Recently, the fractures have been classified by location on the acromion based on the involvement of the attachment of the heads of the deltoid. Type 1 involves the anterior and less than the complete middle deltoid, type 2 involves all the middle deltoid but not the complete deltoid, and type 3 involves the entire posterior deltoid [37]. There needs to be a high index of clinical suspicion for acromion fracture, because routine screening radiographs do not reliably demonstrate the fracture. Any significant pain along the scapular spine or acromion should heighten the awareness of the surgeon to the possibility of an acromion fracture, especially in the setting of a patient with initially good results which acutely deteriorate in pain or function. The fracture may occur in the early postoperative period or years after RSA implantation. If after careful evaluation of the postoperative radiograph, paying particular attention to the axillary film (Fig. 29.4), there is no acromion fracture seen, a CT scan will identify the fracture [37]. However, the diagnosis may be clinically based on presentation and localized tenderness along the acromion.