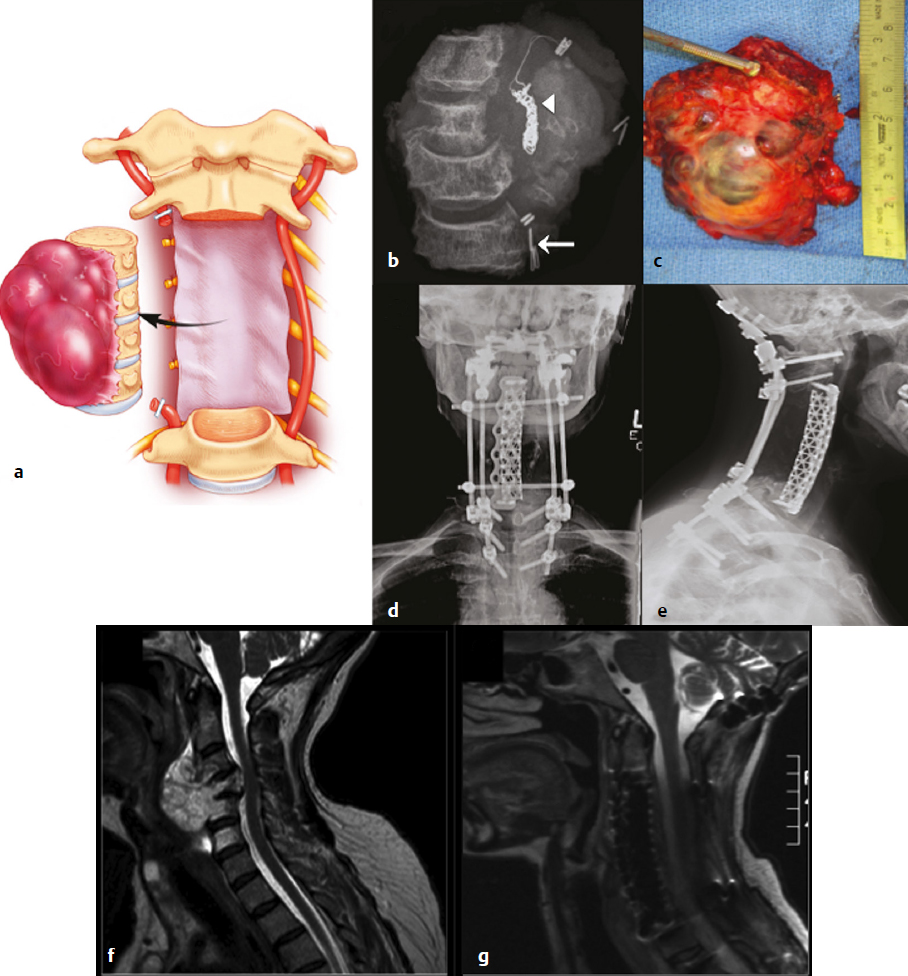
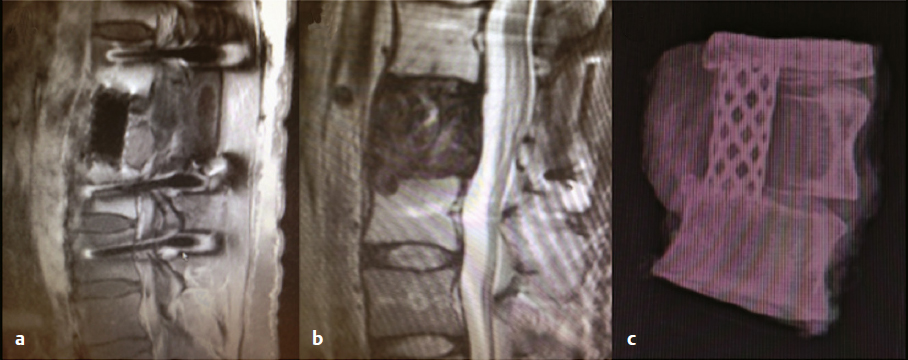
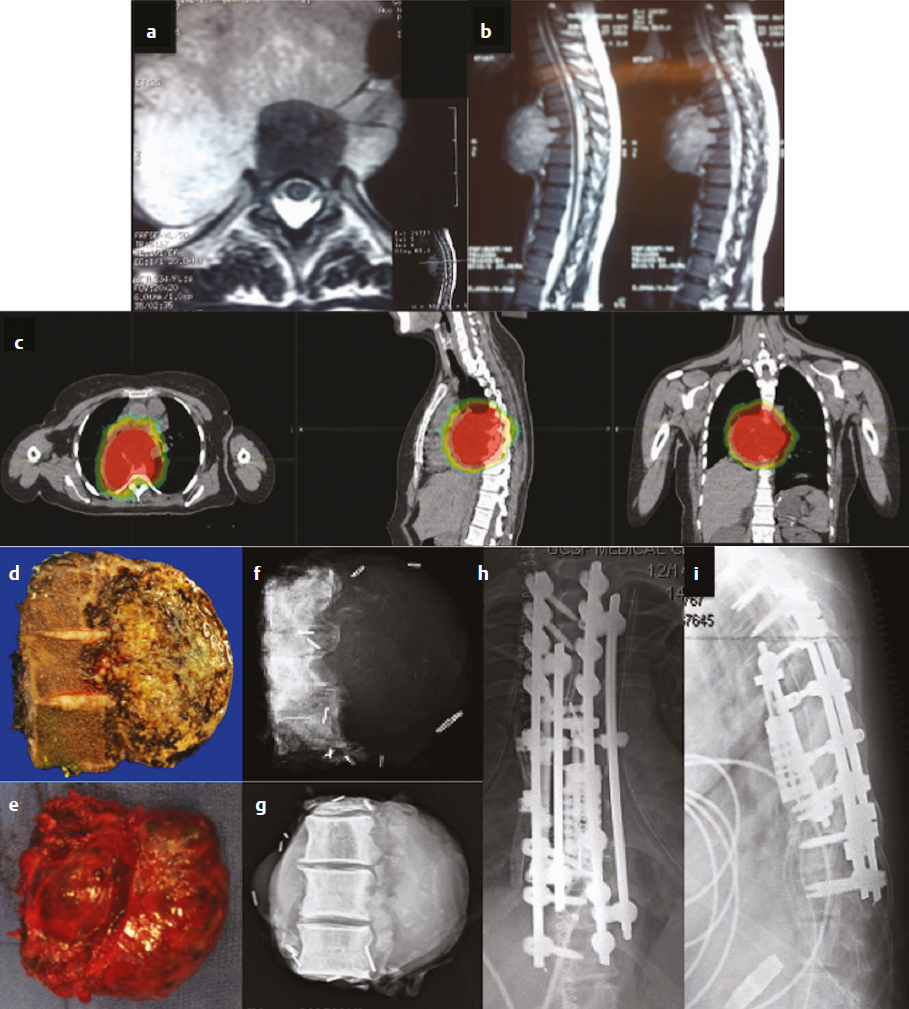
16 Although primary tumors of the spine are rare, their treatment requires some of the most demanding and complex surgical approaches and can result in significant morbidity and mortality.1 The best chance of cure or minimization of recurrence often requires total en-bloc resection for malignant tumors, whereas some benign tumors may be amenable to intralesional piecemeal removal. Due to technical challenges required for wide-margin en-bloc resection with complex reconstruction, complication rates remain high. These complications include construct failure, alignment failure, pseudarthrosis, infection with or without skin dehiscence, spinal cord or nerve root injury, cerebrospinal fluid leak with pseudomeningocele, excessive bleeding requiring transfusions, large-vessel injury, and intraoperative contamination of malignant tumors. Large tumors may involve nearby nerve roots or dura, with planned and unplanned risk of neurologic deficits; wide-margin en-bloc resection for malignant primary tumors may require sacrifice of neural elements to achieve the best chance of recurrence-free cure. Depending on the location of the tumor, different vital anatomic structures may be involved including vertebral arteries, the trachea, esophagus, aorta, vena cava, iliac vessels, ureters, and parts of the distal gastrointestinal tract. Other common postoperative complications, such as deep venous thrombosis, pulmonary embolism, urinary tract infection, pneumonia, dysphagia, ileus, and myocardial infarction may be caused by prolonged immobility and extensive surgical resections with long operative times. Because of the complexity involved in resecting primary spine tumors, careful preoperative planning is mandatory, which includes imaging, biopsy, and, perhaps most importantly, detailed patient counseling and shared decision making. Patients should be well informed of all potential complications, and discussions regarding the risks and benefits of the potential sacrifice of neural elements for the best chance of cure should be part of every preoperative counseling. This chapter reviews the literature related to complications in surgical resection of primary spine tumors and discusses how best to plan primary spine tumor resection to minimize complications while maximizing outcome. We present several cases from our own experience with primary spine tumors to illustrate some of the more common complications as well as the importance of judicious preoperative planning. Planning of primary spine tumor resection starts with a thorough imaging evaluation. Standing scoliosis X-rays should be utilized to assess the regional and global alignment. This is especially important for lumbar tumors, where the postoperative pelvic incidence (PI) should measure within 11 degrees of lumbar lordosis. The location of the individualized thoracic apex should be noted to ensure that thoracic instrumentation extends beyond the apex when planning thoracic resections. Cervicothoracic kyphosis should be noted when planning cervical resections as well. Because primary tumor patients treated with en-bloc resections often have long survival, conforming to deformity principles is important to optimize functionality and patient-reported satisfaction, and to prevent construct failure. Computed tomography (CT) is used to evaluate the extent of bony invasions, but is also critical in evaluating the bone qualities of adjacent vertebral bodies for potential instrumentation. The number of involved vertebral levels should be carefully noted. Whether the tumor is confined to the vertebral body or extends into pedicles or laminas should be evaluated and graded by the Weinstein-Boriani-Biagini (WBB) surgical staging system.2 For example, whether one or both pedicles are involved can change surgical planning, as the sequence and location of osteotomies for total en-bloc spondylectomy differ depending on the extent of tumor invasion into pedicles and posterior bony structures.2,3 The delivery corridor for the specimen should be decided based on the size and location of the bulk of the mass and its adherence to surrounding structures such as viscera and major vessels. Magnetic resonance imaging (MRI), specifically T1-weighted scans with gadolinium or T2-weighted scans, can provide additional information regarding the extent of tumor invasion. Involvement of non-bony structures, such as the epidural space, neuroforamina, or paravertebral tissues, should be carefully evaluated. Although routine preoperative angiograms are not necessary in most cases, selective arterial embolization may provide good outcomes in patients with aneurysmal bone cysts,4 especially given the finding that surgery can result in 15 to 30% complication rates.5 Preoperative angiograms in patients with large cervical tumors may be helpful in evaluating the patency of vertebral arteries (VAs).6 Occlusion tests can also be performed to determine if it is safe to sacrifice the artery, and if sacrificing the VA will assist in tumor resection with wide margins. Coils should be placed distal and proximal to sites of intended VA ligation to prevent inadvertent coil removal and back bleeding at surgery. An example of such case is depicted in (Fig. 16.1). In this patient with giant cervical chordoma spanning four levels from C3 to C6, an angiogram was first performed to coil and sacrifice the right VA, which was encased by the tumor. This was followed by intraoperative ligation of the right VA during the first portion of the procedure via a posterior approach. Following posterior osteotomies and ligation of nerve roots, a Silastic sheet was placed between the dura and the posterior longitudinal ligament to provide an easier plane of dissection for the en-bloc removal of tumor from consequent anterior approach. Proper surgical planning requires biopsy for pathological diagnosis. Although small asymptomatic benign tumors may be observed, symptomatic benign tumors may require surgery for decompression or stabilization.2 For malignant tumors, a biopsy sheath should be used with the tract carefully noted and marked, as enbloc resection requires resection of the biopsy tract for the best outcome. The study by Fourney and colleagues7 suggested that surgery that was performed at the same center where the initial biopsy was done had better outcomes. Although not statistically significant, patients who received biopsy and surgery at the same referral center were 78% disease free at the final follow-up, whereas those whose biopsy was done at one center and who were then referred elsewhere for surgery were 55% disease free at final follow-up.7 Consistent with these results, patients treated for sacral chordomas by biopsy and surgery at the same referral center had a 75% disease-free rate, whereas those initially treated elsewhere had a 42% disease-free rate. Although this finding was also not statistically significant, experts generally agree that biopsy and surgery should be performed at the same institution, preferably one with extensive experience.1,7 Following complete imaging and biopsy studies, a systematic surgical staging should be done to formulate consistent surgical planning. We recommend the use of the WBB surgical staging system2 to help guide more consistent surgical planning and for systematic reporting of surgeries and the extent of tumor invasion. Ewing’s sarcomas, require wide en-bloc resection with adherence to oncological principles for best outcomes.1,2,8–10 For example, en-bloc resection with wide margins provides good long-term local control for 92.3% of giant cell tumors,11 78% of chordomas,8 and 82% of chondrosarcomas.9 By comparison, intralesional resection provides local control for 72.2% of giant cell tumors,11 22% of chordomas,8 and 0% of chondrosarcomas.9 Although it is clear that the best outcomes are provided by en-bloc resection, morbidity remains high, with an overall complication rate of 35.1% with 2.2% overall mortality.1 Certain benign tumors, namely giant cell tumors (GCTs) with Enneking stage III2,11 or posteriorly located aneurysmal bone cysts,4 also have been shown to have the best outcome with en-bloc resection. One such patient in our experience was a 29-year-old man who was previously treated in a different country for a T12 GCT with laminectomy, partial anterior corpectomy, and anterior and posterior spinal instrumentation (Fig. 16.2). A recurrence of this tumor, just lateral to the anterior cage, was noted on follow-up MRI. We performed en-bloc spondylectomy via thoracotomy, which included the recurrent tumor as well as the anterior cage from the previous surgery as one piece. Although complete spondylectomy for resection of primary spine tumors was initially described by Roy-Camille’s group12 and by Stener,13 total en-bloc spondylectomy using a thread-wire saw was first developed by Tomita.3,14 This technique has also been extended for treatment of metastatic tumors for selected patients. Development of these new surgical techniques and devices has enabled performing en-bloc spondylectomies in the spine. Furthermore, the use of a thread-wire saw has been shown to minimize tumor contamination during en-bloc resections.14 Future development of new devices, such as the thread-wire saw guide and spinal cord protector device, may help reduce neurologic deficits and other major intraoperative complications involved with technically challenging total enbloc spondylectomies. Some total en-bloc spondylectomies require multidisciplinary approaches with multistaged operations (Fig. 16.3). In a 38-year-old woman with a giant chordoma spanning from T5 through T8, we performed a three-stage enbloc resection with assistance from thoracic surgeons to dissect and mobilize the aorta and esophagus. The patient was first treated preoperatively with stereotactic radiosurgery with Cyberknife (2,500 cGy), followed by posterior decompression and osteotomies to release the posterior components. The second stage consisted of a left thoracotomy for dissection of the aorta off the tumor mass with anterior spinal osteotomies at T5 and T8-9. The final stage consisted of dissection and mobilization of the esophagus with en-bloc spondylectomy of T5-T8 and delivery of the tumor. The patient is currently recurrence-free 18 months after surgery and lives a very active life. Fig. 16.2a–c En-bloc resection of a recurrent giant cell tumor with previous instrumentation in a 29-year-old man who was previously diagnosed with a T12 tumor and treated at another hospital with a laminectomy, partial anterior corpectomy, and anterior and posterior spinal fusion and instrumentation. Biopsy at the time confirmed giant cell tumor. (a) Preoperative MRI. (b) On follow-up MRI, recurrence was evident lateral to the anterior cage. (c) Thus, we performed an en-bloc spondylectomy via a posterior osteotomy and then a thoracotomy with T10 rib resection, followed by T12 anterior spinal osteotomy, L2-L3 diskectomy, and en-bloc corpectomy of T12-L2. Careful planning allowed salvage of this patient with negative surgical margins. Finally, the spine was stabilized with cage reconstruction from T12 to L3 and anterior spinal fusion and instrumentation from T11 to L3. Decision making regarding the use of different approaches and multidisciplinary teams, which may include head and neck surgery, thoracic surgery, general surgery, and plastic surgery, are also determined by tumor location. Although the evaluation of tumor extension into surrounding structures is a critical step in preoperative planning, the tumor location (cervical, thoracic, lumbar, or sacral) presents inherent risks to different anatomic structures. Primary tumors of the cervical spine present risks to the VAs, esophagus, trachea, cervical nerve roots, and spinal cord. The aorta, esophagus, and vena cava are at risk in the thoracic spine during surgery. Late aortic dissection causing paraplegia and death has been reported following thoracic tumor surgery.1 Lastly, surgery in the lumbar and sacral regions presents risks to the cauda equina, aorta, iliac vessels, ureters, and distal gastrointestinal tracts. In one institutional series by Zileli and colleagues,6 66 surgeries were performed in 35 patients with primary tumors of the cervical spine. There were eight complications, both early and late, including graft extrusion, graft donorsite infection, instrument failure and revision, respiratory complication, diabetes insipidus of unknown etiology, postoperative epidural hematoma, seventh nerve palsy, and one postoperative death. Of note, there was one VA bleeding during tumor resection, which was repaired without complications. Cloyd and colleagues10 performed a systematic review of the literature to identify patients who underwent en-bloc resection for primary cervical spine tumors. Eight patients had complications. The most common complication was dysphagia. Other complications included pneumonia, respiratory distress, seizure, thrombotic event, and wound dehiscence. Of note, mean operative time, estimated blood loss, and length of stay were 18.6 hours (range, 4.3–56 hours), 2.9 L (range, 0.6–8.7 L), and 34.6 days (range, 16–54 days), respectively. From this study, one can conclude that en-bloc resection of cervical spinal tumors carries a high morbidity rate, and thus, patients should be carefully selected and should be well informed about the risks and benefits of surgery. Moreover, due to vital anatomic structures in the cervical spine, wide enbloc spondylectomies, although achievable, are often difficult in the cervical spine6,10 (Fig. 16.1).
Complications and Their Avoidance: How to Plan Primary Tumor Resection to Minimize Complications and Maximize Outcome

 Introduction
Introduction
 Preoperative Planning and Imaging
Preoperative Planning and Imaging
 Preoperative Diagnosis and Biopsy
Preoperative Diagnosis and Biopsy
 Surgical Nuances
Surgical Nuances
Extent of Resection
Tumor Location
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree