Chapter 44 Clinical Genetics (Genomics)
Overview
Genetics vs. Genomics
The terms genetics and genomics are often used interchangeably in the literature, and this chapter is no exception. However, genetics is best viewed as the study of single genes, what they do, and how mutations in these genes cause disease. It is a snapshot of a specific situation, and environmental and behavioral factors often play a subordinate role. A prototypical genetic condition, cystic fibrosis, is usually caused by a deletion mutation ΔF508 in the CFTR gene. Genomics is the broader study of “the functions and interactions of all the genes in the genome,” including how those genes interact with environmental and behavioral factors (Guttmacher and Collins, 2002). When considering genomics, it is important to recognize that environmental factors may be much more important in determining phenotype than genetic mutations. Common, so-called complex conditions, such as diabetes, cancer, and heart disease, are best considered from the perspective of genomics.
Genetics and Evidence-Based Medicine
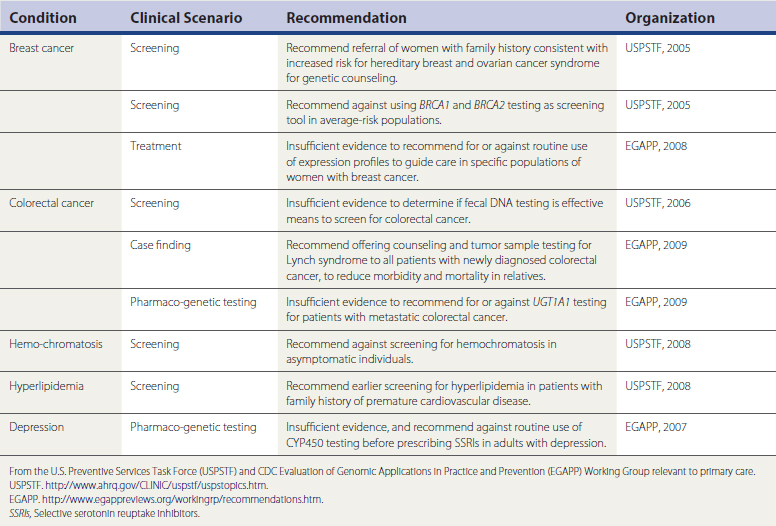
As a discipline, clinical genetics developed in an environment that focused on the diagnosis and treatment of rare, or at least uncommon, disease. Often, large numbers of patients were not available for clinical trials, and in many cases the severity of the conditions made randomized placebo-controlled trials (RCTs) untenable. This contrasts with evidence-based medicine (EBM), which primarily deals with common conditions and values large, prospective RCTs, as well as the societal consequences of individual medical choices. As genomic discoveries increasingly impact health care for common conditions, new applications should be evaluated through a lens of evidence of benefit. Established groups that follow EBM precepts, such as the U.S. Preventive Services Task Force (USPSTF, 2009), have addressed screening and testing for hereditary breast and ovarian cancer syndrome and hemochromatosis. The U.S. Centers for Disease Control and Prevention (CDC) has established newer groups such as the Evaluation of Genetic Applications in Practice and Prevention (EGAPP, 2009) specifically to review the evidence supporting genetic and genomic applications intended for health care use. Modeled after USPSTF, EGAPP has begun to produce evidence reviews and recommendations (Table 44-1). Considerable growing pains will occur as EBM and genomic medicine intersect.
Family History: Best Guide to Genetic Components of Disease
Family history is arguably the single best tool for recognizing genetic components of disease in the primary care setting. In the context of single-gene disorders, family history has proved valuable for generations of clinicians and plays a major role in making a diagnosis and identifying at-risk individuals. Family physicians should be familiar with common patterns of inheritance of single-gene disorders, including X-linked recessive, X-linked dominant, autosomal dominant, autosomal recessive, and multifactorial/complex (Table 44-2). Classically, the three-generation genetic history known as a pedigree or genogram has been taught as the “gold standard” of family history collection. Certainly, once a potential genetic issue has been identified, a family physician should be comfortable in collecting and accurately representing a complete family history. However, taking a complete family history can be time-consuming, and on a practical level, it is not always possible to collect in the context of a brief office visit. It is perfectly reasonable to gather, review, and update family history longitudinally.
Table 44-2 Patterns of Inheritance Often Encountered in Primary Care
| Pattern of Inheritance | Characteristics Of Family History | Example Conditions |
|---|---|---|
| X-linked recessive | Males affected more than females, maternal inheritance, 50% risk of female carrier sons affected | |
| X-linked dominant | Males and females may be affected, males more severe, daughters of affected males affected, male and female transmission | Fragile-X syndrome |
| Autosomal dominant | Affected individuals usually in every generation, 50% probability of affected individuals having affected offspring, M = F | |
| Autosomal recessive | Often multiple affected individuals in same generation, skipped generations, 25% risk of affected child for carriers, M = F | |
| Multifactorial | Clustering of cases in families, risk to first-degree relatives high; consequences of shared environment might be evident. |
Electronic health record (EHR) systems seldom offer efficient and complete ways to collect and represent family history information. National efforts are underway to address this deficiency. Patient-completed paper and electronic tools provide another way to obtain a detailed genetic history. The U.S. Surgeon General’s Family History Initiative (2005) includes a web-based tool that can be completed by patients, stored on their local computer, and shared with relatives and their health care providers in pedigree or table format (My Family Health Portrait; https://familyhistory.hhs.gov/). This free, easy-to-use tool is an excellent way for patients with Internet access to record family history and is time-saving for the clinician. The family history collected by the tool is now stored using emerging data standards that allow the data to be shared with EHR and personal health record systems. Alternatively, a number of organizations have created paper family history tools for patients and providers that are available on the Internet.
Genetic Testing
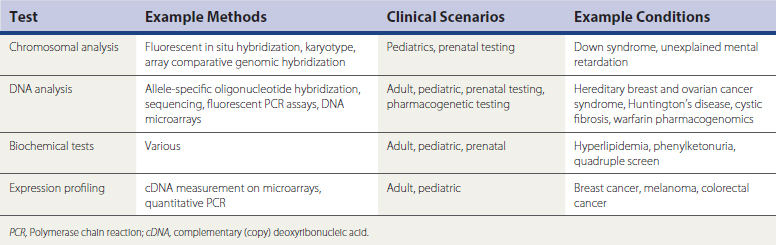
Identifying what constitutes a genetic or genomic test can be challenging. Traditionally, a genetic test measures changes in the sequence of deoxyribonucleic acid (DNA), but a “genetic test” can also be a measure of a protein or metabolite (Table 44-3). From this perspective, a fasting lipid panel could be considered a genetic test. Also, a genetic test does not always need to be relevant to other family members, as when an individual’s cancer cells are tested for mutations that affect prognosis and therapy. In some cases a family physician’s most important role is simply to reassure low-risk individuals that they do not need genetic testing.
Examples of Genetic Testing
Preconception and Prenatal Screening
Genetic screening or testing can occur either before conception or during the pregnancy. When possible, screening or testing before a pregnancy is ideal, because this provides the broadest range of choices if increased risk of a genetic defect is detected. Preimplantation genetic testing is available for an increasing number of conditions but is costly and not accessible to many individuals. Most genetic evaluations occur after the pregnancy is established. The ability to detect genetic defects has grown rapidly over the past decade. Many ethical issues exist in both the preconception and the prenatal screening or testing environment, including the course of action if the fetus is found to have an incurable, life-altering condition. Common indications for prenatal testing are advanced maternal age, previous child with a chromosomal abnormality, family history of abnormality or single-gene disorder, family history of neural tube defect or other structural abnormality, abnormalities identified in pregnancy (e.g., on ultrasound), parental consanguinity, recurrent miscarriages, previous unexplained stillbirth, parental ancestral origin, and use of certain medications. The American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Geneticists (ACMG) have developed guidelines for recommended tests (Solomon and Feero, 2008). However, these guidelines are often based on consensus or expert opinion and do not always agree.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree