Clinical Approach to Articular Cartilage Pathology
Andrea L. Bowers
Thomas L. Wickiewicz
Articular cartilage pathology in the knee is commonly encountered and may vary significantly in etiology and symptomatology and therefore management. Many cartilage irregularities are incidental findings and may be best treated with observation. Others are a source of significant pain and mechanical symptoms that warrant surgical intervention. Decision making in the management of articular cartilage lesions depends on a multitude of factors including lesion-specific etiology, location, chronicity, and size, concomitant pathologies, as well as patientspecific age, activity level, physical demands, and rehabilitation potential. A thorough understanding of articular cartilage composition, function, and injury patterns as well as a comprehensive clinical evaluation is necessary for successful management of such lesions.
STRUCTURE, FUNCTION, AND METABOLISM OF ARTICULAR CARTILAGE
Articular cartilage is a complex, highly organized biologic lining of the ends of articulating surfaces. In the knee, it surfaces with varying thickness the underside of the patella, trochlea, femoral condyles, and tibial plateau. Its main functions are to provide a smooth, durable surface for near-frictionless gliding and to withstand and distribute forces transferred across the joint.
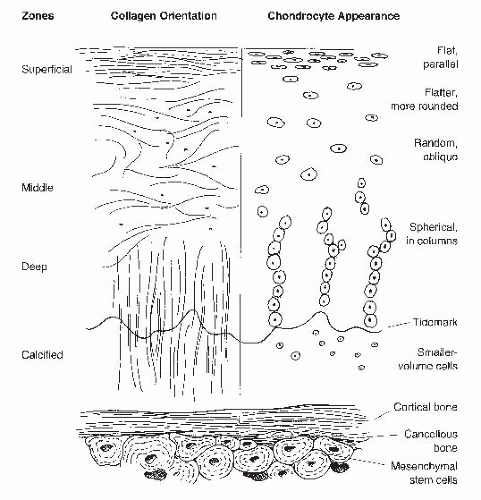
Histologically, articular cartilage is composed of distinct layers (Fig. 63.1). The superficial 10% to 20%, called the tangential zone, consists of flattened chondrocytes and collagen fibers lined parallel to the joint surface. In the intermediate 40% to 60%, or transitional zone, cells are round and sporadically dispersed among obliquely oriented collagen fibers. The deepest 30%, comprising the basal zone, features more densely packed chondrocytes aligned in vertical columns. Proteoglycan concentration is greatest in this zone. Collagen fibrils extend from the basal zone across a tidemark, which separates this deep layer from the underlying calcified cartilage, and ultimately subchondral and cancellous bone.
The cartilage function is a reflection of its intricate morphology and biologic activity. This avascular, alymphatic tissue is a composite of chondrocytes suspended within a collagen and proteoglycan-rich extracellular matrix. Chondrocytes, which comprise 1% to 10% of articular cartilage volume, are derived from undifferentiated mesenchymal marrow stem cells. Chondrocytes maintain matrix homeostasis through synthesis and degradation of matrix material in response to local tissue composition, growth factor and cytokine effect, mechanical load, aging, and injury. Another 60% of cartilage dry weight is composed of collagen, 90% of which is highly cross-linked type II fibrils, and the remainder types IX and XI. This collagenous portion contributes to the form and tensile properties of articular cartilage. The ability to withstand compression, however, is attributed to proteoglycans, which comprise
the remaining 35% of the tissue’s dry weight. Proteoglycan structure includes a core protein, aggrecan, which is produced by chondrocytes and is unique to hyaline cartilage. A single aggrecan unit can have up to 60 keratin sulfate and 100 chondroitin sulfate glycosaminoglycan chains attached; these sulfated disaccharides lend a large negative charge to the aggrecan molecule. Aggrecan strands are polar, and their N-terminal globular domains attach, or aggregate, through link proteins to a hyaluronic acid chain. The negatively charged proteoglycans interspersed within the collagen matrix increases tissue osmolality, thereby attracting water molecules. Water comprises 75% to 80% of the wet weight of articular cartilage. The interplay of proteoglycans and water in maintaining an osmolal balance allows the tissue to withstand high pressures seen with compression. Swelling of the tissue is in turn prevented by the highly organized type II collagen matrix. The matrix is further stabilized by smaller proteoglycan units including decorin, biglycan, and fibromodulin; greater concentrations of these smaller, stabilizing proteoglycans have been observed in response to increased physiologic stress (1).
the remaining 35% of the tissue’s dry weight. Proteoglycan structure includes a core protein, aggrecan, which is produced by chondrocytes and is unique to hyaline cartilage. A single aggrecan unit can have up to 60 keratin sulfate and 100 chondroitin sulfate glycosaminoglycan chains attached; these sulfated disaccharides lend a large negative charge to the aggrecan molecule. Aggrecan strands are polar, and their N-terminal globular domains attach, or aggregate, through link proteins to a hyaluronic acid chain. The negatively charged proteoglycans interspersed within the collagen matrix increases tissue osmolality, thereby attracting water molecules. Water comprises 75% to 80% of the wet weight of articular cartilage. The interplay of proteoglycans and water in maintaining an osmolal balance allows the tissue to withstand high pressures seen with compression. Swelling of the tissue is in turn prevented by the highly organized type II collagen matrix. The matrix is further stabilized by smaller proteoglycan units including decorin, biglycan, and fibromodulin; greater concentrations of these smaller, stabilizing proteoglycans have been observed in response to increased physiologic stress (1).
Cell surface-binding proteins termed integrins link chondrocytes to the extracellular matrix and allow the cells to be stimulated by mechanical forces imparted to the matrix. Cellular metabolism, including matrix secretion and degredation, is thereby regulated in great part by local forces. As articular cartilage is devoid of vascular and lymphatic channels, oxygen and nutrients needed to maintain homeostasis are obtained instead by diffusion from synovial fluid. Due to low local oxygen tension, anaerobic metabolism of glucose through glycolysis serves as the major source of fuel for chondrocytes. Metabolites accumulate in the interstitial fluid, which is expressed from the permeable collagen-proteoglycan matrix in response to a compressive load. When the compression is withdrawn, the matrix is reconstituted with nutrient-rich fluid. Further, metabolic activity in the chondrocyte stagnates in the face of static pressure but is upregulated in response to changes in hydrostatic pressure seen with dynamic loading. Mechanical stimulation is therefore paramount to maintaining chondrocyte health.
CARTILAGE INJURY AND HEALING RESPONSE
The lack of direct blood supply also impedes the articular cartilage’s ability to mount a healing response in the face of injury. Spontaneous repair is seldom seen without violation of the tidemark, which allows egress of reparative cells originating from subchondral bone. Partial-thickness cartilage injury, observable only on the microscopic level, is frequently the result of a blunt trauma imparting a compressive load that exceeds the tissue’s tolerance. Local cells undergo apoptosis. A transient metabolic and enzymatic response leads to collagen degradation and proteoglycan loss. A zone of necrosis develops that does not remodel over time (2). Partial-thickness injury may also result from repetitive microtrauma. Chronic increased stress affects thinning of the cartilage and, in response, a thickening of the calcified cartilage layer. Since the cartilage layer is aneural, pain signals are not appreciated until enough microdamage has accumulated to expose underlying subchondral bone.
A full-thickness cartilage injuries can be limited to the cartilage itself (transchondral fracture) or extend to the underlying subchondral bone (osteochondral fracture). Chondral fractures, like partial thickness injuries, result in chondrocyte necrosis and apoptosis. Surviving cells attempt to repair the tissue by proliferating into clusters and upregulating collagen and matrix production at the periphery of zone of injury. This response is short lived, however, and fails to bridge the chondral fracture (3). Propagation of the injury over time damages a greater surface area and leads to progressive and symptomatic joint deterioration.
CLASSIFICATION OF ARTICULAR CARTILAGE LESIONS
The most commonly used classification system for articular cartilage lesions was described by Outerbridge (4) in 1961 for grading of patellar lesions. Four grades of progressive size and depth of injury by gross appearance are described. Many employ a “modified” Outerbridge classification (Fig. 63.2), which describes partial-thickness fissuring as grade 2 and full-thickness fissuring as grade 3, regardless of size of area involved. Grade 4 injuries result in exposure of subchondral bone.
A newer classification system has been set forth by the International Cartilage Repair Society (ICRS), founded in 1997 to facilitate collaborative research endeavors (5). The ICRS recommends specific systems for articular cartilage injury mapping, articular cartilage injury classification, osteochondritis dissecans (OCD) classification, and a cartilage repair assessment system. The articular cartilage injury classification involves five grades, and the OCD classification four grades (Table 63.1).
Regardless of the classification system employed, depth, size, shape, location, and quality and morphology of the shouldering tissue are all important factors to note when describing cartilage lesions and determining a management plan.
INCIDENCE
A great challenge in the evaluation of articular cartilage lesions is determining which lesions are incidental findings versus true symptom generators. A retrospective review of more than 30,000 knee arthroscopies identified a 63% incidence of articular cartilage lesions, most commonly involving the patella and the medial femoral condyle. Forty-one percent were Outerbridge grade 3 and 19.2% Outerbridge grade 4 chondral injuries (6). With
vast improvements in MRI in the past two decades, articular cartilage can now be scrutinized with great detail. The goal is to be able to detect, through history, physical examination, and MRI evaluation, clinically relevant cartilage injuries requiring treatment.
vast improvements in MRI in the past two decades, articular cartilage can now be scrutinized with great detail. The goal is to be able to detect, through history, physical examination, and MRI evaluation, clinically relevant cartilage injuries requiring treatment.
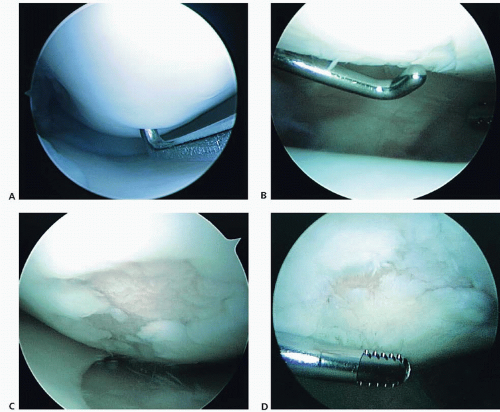 FIGURE 63.2. Arthroscopic photographs of articular cartilage lesions using modified Outerbridge classification. A: Grade 1 softening. B: Grade 2 partial-thickness fissuring. C: Grade 3 full-thickness fissuring and fibrillation. D: Grade 4 articular changes with exposed bone centrally surrounded by diffuse grade 3 articular cartilage.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|