Adolph V. Lombardi Jr.
Calcar Replacement Stems
INTRODUCTION
The primary goal of total hip revision arthroplasty is to reduce pain and maximize functional capacity. This requires obtaining stable long-term femoral fixation. In revision arthroplasty, the femoral bone stock is generally compromised by a combination of stress shielding, osteolysis, and iatrogenic damage from implant removal. The quality and quantity of bone in the femoral neck region and proximal to the calcar femorale are routinely inadequate to sufficiently support primary femoral components for use in the new prosthetic construct. Early cementless revision prostheses tended to be longer versions of primary.1,2 Because the proximal femoral bone quality and support needed to establish fixation are absent, these longer versions tended to fail from loosening and subsidence. These concerns led to the development of the cementless calcar replacement prosthesis.
The concept of the calcar prosthesis dates back to the early work by Leinbach. The Leinbach head/neck prosthesis was initially utilized for the treatment of peritrochanteric fractures, failed open reduction internal fixation of peritrochanteric fractures, comminuted proximal femur fractures, and pathologic fractures. The results of this early cemented device were encouraging in these difficult cases.3 The Leinbach device was further modified by Harris who reported longterm results on a cemented calcar replacement prosthesis with a mean follow-up of 10.8 years. The authors identified an 18% revision rate for aseptic loosening with an additional 11% incidence of radiographic loosening. Cognizant of early and late failure with cemented revision arthroplasty4–7 and concomitant with the success of cementless femoral fixation in the primary application, a cementless calcar replacement prosthesis was designed.
The cementless calcar replacement prosthesis is the authors’ prosthesis of choice for almost all femoral revisions, and the authors of this chapter favor the device in Mallory bone loss Type II and III femora. The Mallory classification categorizes the femur by both the integrity of the corticocancellous junction and the cortical tube itself (Fig. 24-1).8 Type I femora have an intact corticocancellous junction and cortical tube. In these cases, revision to a cementless or cemented primary femoral stem is possible. Type II femora have a deficient corticocancellous junction with an intact cortical tube and are subdivided into Type IIA and IIB based on the quality of the cortical tube proximal to the calcar femorale. In Type IIA, the cortical tube is satisfactory, and in Type IIB, the cortical tube proximal to the calcar femorale is thin, atrophic, or deficient. Type III femora have a deficient corticocancellous junction and varying degrees of deficiency of the cortical tube. In Type IIIA, the cortical tube is deficient to the level of the lesser trochanter, in Type IIIB, the cortical tube is deficient to the level of the metaphyseal-diaphyseal junction, and in Type IIIC, the cortical tube deficiency extends to the diaphyseal region. Type II and Type III femora require revision prostheses rendering the calcar prosthesis, the implant of choice at our institution. This is especially true for the Type III femora, which may also require supplemental bone grafting. Cortical strut allografts in combination with the calcar prosthesis have consistently demonstrated excellent femoral bone reconstitution (Fig. 24-2).9–11 There are many manufacturers with cementless calcar-type femoral revision prostheses available on the market today.
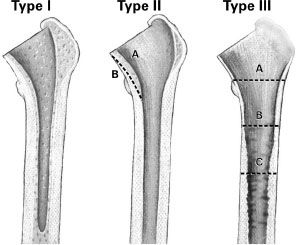
FIGURE 24-1. The Mallory classification of femoral deficiency and deformity in revision THA is shown. (Reproduced courtesy of Joint Implant Surgeons, Inc.)
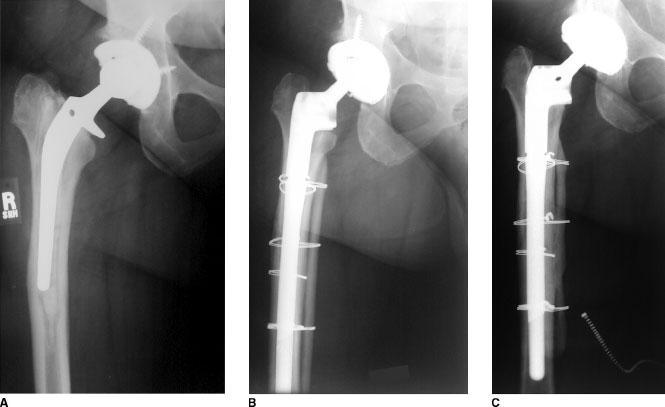
FIGURE 24-2. Revision THA performed for failed cemented primary THA (Mallory Type III-A femur) in a 53-year-old woman. A: Radiograph preoperative to revision THA demonstrates a femur classified as Mallory Type IIIA. B: Immediate post-operative radiograph demonstrates reconstruction using a Mallory-Head Modular Calcar femoral component (Biomet, Warsaw, IN) augmented with strut allograft and cerclage wires. C: Radiograph at 40 months following revision THA shows evidence of a well-fixed and stable femoral composite. Note incorporation of medial onlay strut allograft. At the most recent follow-up, the patient’s Harris hip score im-proved from 54 preoperatively to 84.
The authors utilize the Mallory-Head Calcar prosthesis (Biomet, Warsaw, IN) in these revision cases (Fig. 24-3). The calcar implant design features titanium as the substrate metal, a material advantageous for its modulus of elasticity and optimization of load transfer to bone. Additionally, titanium generates an oxidation layer on its surface when exposed to blood and fluid. This oxidation layer creates a dielectrically charged current that stimulates osteosynthesis and encourages bone ongrowth to the stem.12 A circumferential coating of plasma spray is applied to effect an endosteal seal and further stimulate biologic fixation.13

FIGURE 24-3. Photographs show the Mallory-Head Calcar prosthesis (Biomet, Warsaw, IN), available as a monoblock (A) or modular (B) design.
Successful, long-lasting bony fixation is accomplished via four synergistic design parameters. The first is through direct platform loading of the calcar replacement prosthesis onto the medial and posterior medial bone of the revision femur. The second fixation mode is via the tapered proximal third of the stem and its associated titanium porous plasma spray osseointegration surface. The longstem design allows for a tight distal diaphyseal fit with a cylindrical-type stem, thus providing the third modality of fixation. Lastly, compressive force applied to the trochanter by a trochanteric plate or claw, when utilized, provides a fourth mode of rotational and axial stability.
The cementless Mallory-Head Calcar prosthesis was available originally as a monoblock device and is now available in both a monoblock and modular system (Fig. 24-3). The modular system was designed to accommodate proximal metaphyseal and distal diaphyseal mismatch via a variety of modular implant size options. Through modularity, the surgeon is able to maintain the proximal fixation modalities while increasing distal fixation though various stem lengths and degrees of porous coating. In the revision scenario, the surgeon is presented with varying degrees and severity of proximal bone loss. To accommodate these varying levels of bony deficiency, several heights of calcar replacement are currently available. In very large patulous femora, which require stem diameters in excess of 20 mm, long cylindrical modular stems may not provide sufficient fixation. In these uncommon cases, a large-diameter splined tapered stem can be utilized in combination with the modular calcar. This provides the advantage of rotational stability and bony fixation with the splined stems and control of subsidence with loading of the calcar region by the prosthetic platform.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








