Lower respiratory tract (Figure 5.1)
Larynx
The larynx lies below the pharynx and in front of the oesophagus. It consists of a series of cartilages connected by muscles and ligaments and lined with ciliated mucous membrane. The largest of these cartilages is the thyroid cartilage (Adam’s apple). The thyroid gland lies on either side of the thyroid cartilage.
On the superior edge of the larynx is a leaf-shaped cartilage – the epiglottis which covers the larynx during swallowing and thus prevents food entering the trachea or lower airway. The lowest part of the larynx contains the vocal cords. Air from the chest causes vibration of the vocal chords. The sound created is modified by the tongue, palate and lips.
Trachea, bronchi and bronchioles
The trachea (windpipe) is ~12-cm long. It extends into the chest cavity in front of the oesophagus. It consists of a series of C-shaped rings of cartilage connected by smooth muscle, and is lined by ciliated mucous membrane. The C-shaped rings maintain the lumen of the trachea, thus ensuring a patent airway. The ‘open’ part of the C lies posteriorly, next to the oesophagus and allows for some ‘give’ or movement of the oesophageal wall when we swallow.
The trachea divides at its lower end (the carina) to form the right and left bronchi which enter the lungs. The right bronchus is shorter and more vertical than the left which is slightly displaced by the heart. The bronchi divide within the lungs into smaller bronchi and bronchioles. Their structure is similar to that of the trachea, but as the bronchi divide there is less cartilage and more smooth muscle until the smallest bronchioles contain no cartilage at all.
Alveolus
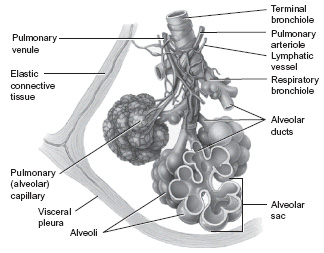
The terminal bronchioles end in a cluster of air sacs called alveoli (singular – alveolus). These bronchioles and alveoli are surrounded by elastic tissue. An alveolus consists of a thin layer of squamous epithelium supported by elastic connective tissue within a thin interstitial space. These have very thin walls consisting of a single layer of cells. It is because of these thin walls that gas exchange can take place. The alveoli are surrounded by the capillaries of the pulmonary circulation. Gaseous exchange takes place between the alveoli and these blood capillaries where oxygen leaves the alveoli to enter the blood capillaries and carbon dioxide leaves these capillaries to enter the alveoli. The carbon dioxide is then exhaled. The movement of gases across the alveolar–capillary membrane takes place by diffusion. Diffusion is the movement of a dissolved substance (the solute) from an area of high concentration to an area of low concentration until there is an equal distribution. The structure and number of alveoli mean that there is a huge surface area across which diffusion takes place.
Lungs
The lungs are conical structures with the point or apex lying under each clavicle and the base resting on the diaphragm. They are divided into lobes – three in the right lung and two in the left. Each lobe is further divided into lobules which contain hundreds of alveoli (Figure 5.2). The bronchi enter the lungs at the hilum which is also the place where the blood vessels and nerves enter and leave. The lungs are supplied with blood from two sources – pulmonary arteries bringing deoxygenated blood from the right ventricle. These vessels go on to form the capillaries which surround the alveoli. It is here that oxygen is taken in and carbon dioxide given off. The other vessels, the bronchial arteries, supply oxygenated blood to the tissues of the bronchioles and lung.
The lungs are stimulated by the ANS. The parasympathetic branch is carried in the vagus nerve. Parasympathetic stimulation leads to vasoconstriction (narrowing) of the bronchioles and sympathetic stimulation leads to dilation (widening) of the bronchioles.
Pleura
This double-layered membrane surrounds the lung tissue. The inner or visceral layer is attached to the outer surface of the lungs. The outer or parietal layer is attached to the undersurface of the ribs and intercostal muscles, and the superior surface of the diaphragm. The two layers of pleura lie close together, but there is a potential space between them. This pleural space is a fluid-filled cavity which allows the pleural layers to move smoothly against one another during breathing.
Figure 5.2 Diagram of lung lobule. (Reproduced from Nair and Peate (2009). With permission from John Wiley & Sons.)
Thoracic cavity
This is the cavity which contains most of the respiratory system. The lungs are contained within the thoracic cavity, which is bounded by the neck at the top, the diaphragm at the bottom and the sternum, ribs, spine and intercostal muscles in the circumference. The diaphragm is a sheet of involuntary muscle which separates the chest from the abdomen. It is dome shaped when relaxed and when contracted it flattens, thus increasing the size of the thoracic cavity. Two sets of intercostal muscles link one rib to the next.
Mechanics of respiration
The two sets of intercostal muscles are the external and internal intercostals. The external intercostals, when contracted, pull the ribs outward and upward. This enlarges the circumference of the thoracic cavity, that is, from front to back. Relaxation of these muscles allows the thoracic cavity to return to its original shape and size. The internal intercostals are only used during forced expiration such as strenuous activity or blowing up a balloon. Contraction of these muscles squeezes more air out of the lungs than is needed during normal respiration, giving a forced expiration.
The mechanics of breathing
Inspiration
For inspiration to occur, the pressure within the lungs must fall below atmospheric pressure. This occurs as a result of the contraction of the diaphragm and external intercostal muscles. This contraction increases the size of the thorax causing the lungs to expand (contraction of the diaphragm increases the size of the thorax from top to bottom, whilst contraction of the external intercostals increases the size of the thorax from back to front). When the lungs expand, the pressure within them decreases (intrapulmonic pressure) due to Boyle’s law, and a pressure gradient is formed. Boyle’s law states that pressure is inversely proportional to volume, that is, as the volume increases within the thoracic cavity, the pressure decreases. Atmospheric pressure is now greater than intrapulmonic pressure, and air rushes into the lungs until intrapulmonic pressure equals that of atmospheric pressure. The process of inspiration is an active one, that is, it requires energy, or to put it another way, breathing is work.
During quiet breathing, the diaphragm and external intercostals relax, decreasing the size of the thorax. The ribcage moves downwards and inwards, and as the diaphragm moves upwards, the size of the thoracic cavity is decreased. The tension is taken off the pleura and the elastic tissue of the lungs recoils. The pressure within the lungs (intrapulmonic pressure) increases and a pressure gradient is formed. Intrapulmonic pressure becomes greater than atmospheric pressure. Air now flows out from the lungs until these two pressures are equal.
Oxygen in the lungs is not yet available to the tissues. External respiration is the process by which gases are exchanged between the alveoli and the blood in the pulmonary capillaries. The process is brought about by the difference in pressures between gases in the alveoli and in the pulmonary capillaries. Atmospheric air is composed of three main gases: nitrogen (N), oxygen (O) and carbon dioxide (CO2). Nitrogen is an inert gas and only the levels of oxygen and carbon dioxide are important in respiration.
Not all the air we breathe in reaches the alveoli. The air passages (nose, pharynx, larynx, trachea, bronchi and bronchioles) contain air which does not reach the alveoli. These parts of the respiratory system which do not take part in gas exchange are referred to as dead space. In adults, the size of this dead space is ~150 ml (Waugh and Grant, 2006).
Expiration
During forceful expiration, the rate and depth of respirations are increased as the expiratory centre is activated. Impulses are now sent to the expiratory muscles – internal intercostals and the abdominal muscles. Both these groups now contract and expiration is more forced than when passive recoil occurs alone. The lungs are prevented from recoiling too far because of the pleura. Since the layers of the pleura remain in contact with one another and the parietal layer is attached to the thoracic wall, the lungs will be limited in the extent to which they can recoil.
During normal quiet breathing, ~400–500 ml of air is inspired and expired. This is called the tidal volume. However, there are times when this amount is insufficient, such as when exercising or when someone has a respiratory disease.
The inspiratory reserve volume is the extra amount of air you can breathe in over and above the tidal volume, that is, after taking a normal breath, continue to breathe in for as long as you can. Men average about 3.3 l as their inspiratory reserve volume and women average about 1.9 l.
The expiratory reserve volume is the extra amount of air you can breathe out over and above the tidal volume, that is, at the end of a breath, breathe out as far as you can. Men average about 1.0 l as their expiratory reserve volume and women average about 0.7 l.
The reason that the figures for inspiratory reserve volume is not the same as for expiratory reserve volume is that the lungs never completely empty. Even after breathing out forcefully, the lungs still contain about 1 l of air. This is called the residual volume.
The inspiratory capacity is the maximum amount of air you can breathe in = tidal volume + inspiratory reserve volume.
The vital capacity is the maximum amount of air you can breathe out after the lungs have been filled to their maximum extent = inspiratory reserve volume + tidal volume + expiratory reserve volume.
The total lung capacity is the vital capacity + residual volume. In men this averages out as 6 l and in women it is 4.2 l.
Gas exchange
As stated earlier, the walls of both alveoli and pulmonary capillaries are very closely associated with one another making diffusion easier. Diseases such as pneumonia cause the alveolar–pulmonary membrane to thicken, making gas exchange more difficult. The structure and number of alveoli mean that there is a huge surface area across which diffusion has to take place.
Oxygen and carbon dioxide transport
Blood returning to the lungs from the right ventricle is deoxygenated blood, that is, it has given up some of its oxygen to the tissues. The pO 2 in this blood is lower than the pO 2 in the alveoli. (Remember, gas at a higher concentration/pressure will move by diffusion to an area of lower concentration/pressure.) Therefore, oxygen moves from the alveoli into the blood.
In contrast, carbon dioxide levels in the blood returning to the lungs are higher than those within the alveoli, and therefore carbon dioxide diffuses from the blood out into the alveoli. It is then expired on the exhaled breath.
Control of breathing
Breathing for the most part is an involuntary action; however, this can be overridden by voluntary control if and when necessary, for example, breathing is under voluntary control when we speak and form words and sentences. The main control of respiration is the result of the interplay of a number of different elements. These include:
- Nervous system control
- Chemical control
- Other factors.
Nervous system control
As with many vital functions, the control of breathing is located in the brainstem, namely the medulla oblongata and the pons varolii.
Within the medulla can be found the respiratory centre which is also known as the medullary rhythmicity centre. This sets the basic pattern of breathing. The respiratory centre can be subdivided into inspiratory and expiratory areas.
The inspiratory centre (primary respiratory pacemaker) exhibits an intrinsic excitability that sends nerve impulses along the phrenic and intercostal nerves. These cause the diaphragm and the external intercostal muscles to contract, thus increasing the thoracic volume. At the same time, the inspiratory centre sends an inhibitory impulse to the expiratory centre, thus ensuring that only inspiration can occur. It is important to note that inspiration is always an active process that requires energy.
The expiratory centre is normally inactive during quiet respiration, and expiration is therefore a passive process dependent upon the elastic recoil of the diaphragm and external intercostal muscles. However, it becomes active when there is a need to increase the rate and/or depth of respiration. When active, impulses travel along the intercostal nerve to the internal intercostal muscles and also to the abdominal muscles. When the internal intercostal muscles contract, the ribcage is pulled downwards and inwards. When the abdominal muscles contract, intra-abdominal pressure increases causing the diaphragm to move upwards. Both of these actions increase the intra-thoracic pressure forcing air out of the lungs. During expiration, inspiration is inhibited. Normal quiet breathing consists of repeated cycles of inspiration, expiration and short pause. Inspiration lasts seconds and expiration 3 seconds (Seeley et al., 2003; Waugh and Grant, 2006).
Chemical control
In addition to the nervous control of breathing, the body also responds to a number of changes in body chemistry.
Chemoreceptors are specialised nerve cells which respond to chemicals that become attached to receptors on their cell membranes. In respiration, these include chemoreceptors in the carotid artery and aorta, and a chemosensitive area in the medulla. The chemoreceptors found in the carotid artery and the aorta are sensitive to changes in blood CO 2 and O2 levels as well as hydrogen ion concentration (H+). However, it should be noted that these chemoreceptors are only sensitive to large decreases in O2 level of ~50%. At this level, hypoxia will then have a large stimulatory effect on respiration.
The medullary chemosensitive area is activated by changes in CO2 and H+, with oxygen having no direct effect on this area. Excess CO2 and H+ stimulate both inspiratory and expiratory centres, thereby increasing the rate and depth of respiration.
Due to the very soluble nature of CO2, the medullary area is able to detect very small changes in this respiratory gas, and thus it is CO2 concentration and not O2 that is the key influence over normal breathing.
It may be useful to consider the following equation:
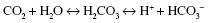
In the body, carbon dioxide will combine with water to produce carbonic acid. This can then break down to produce hydrogen ions and bicarbonate ions.
It can be seen from the above equation that a rise in pCO 2 will result in an increase in H+, thus lowering pH. If pCO2 rises above normal, then the body can compensate by increasing the respiratory rate and depth, thereby increasing the excretion of CO2. This moves the above equation to the left, thus ‘locking’ some of the H+ up in carbonic acid and therefore maintaining blood pH within normal limits.
This reaction is reversible as seen by the double-headed arrows and can occur as:
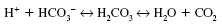
The reaction is facilitated by the enzyme carbonic anhydrase.
It should be noted that the renal system also has a role to play in maintaining a normal pH. It does this by secretion and selective re-absorption of H+ + HCO3”.
Ageing changes in the respiratory system
The respiratory system starts to decline well before the age of 60. A gradual decline is observed in the respiratory system from the age of about 25 years, having reached its functional peak by around the age of 20 years (De Martinis and Timiras, 2003). There are a number of variables that influence the extent and speed of age-related changes in structure and function, which include intrinsic factors such as the presence of disease and extrinsic factors that include nutrition, physical exercise and smoking (De Martinis and Timiras, 2003). However, changes due to ageing are relatively insignificant when compared to the continuous effects of the external environment. If optimal growth in lung function is attained, there should be sufficient spare capacity for a decrease in lung function to have little effect as long as an older adult remains free from disease (Dyer and Stockley, 2006). The majority of older adults are therefore able to sustain their lifestyle and a satisfactory level of respiratory function that meets their needs under resting conditions (De Martinis and Timiras, 2003).
Changes in the structure of the respiratory tract
The respiratory tract is affected by ageing changes associated with the musculoskeletal system. Musculoskeletal changes account for alterations in the size and shape of the nose where the nose becomes longer and its tip begins to sag. This sagging is due to a weakening of the support offered by the upper and lower cartilages. These physical changes can impede the airflow through the nasal passages, as a result of which older adults may experience symptoms of blockage in their nose.
In addition to these musculoskeletal changes, the number of sub-mucosal glands decreases. The upper respiratory tract normally produces a nasal mucus which is thick and tenacious (Sheahan and Musialowski, 2001). This mucus is produced by goblet cells within the respiratory tract. Other glands, called sub-mucosal serous glands, produce a thin and watery mucus. In adulthood, there is a balance between the amount of thick tenacious mucus and thin watery mucus that is produced. However, with ageing, this balance is lost and a larger amount of thick, tenacious mucus is produced while there is a decrease in watery thin mucus production. This results in mucus becoming even thicker and more likely to lodge in the nasopharynx. Older adults may therefore experience recurrent coughs as they try to clear the nasopharynx. Accompanying these changes is a decrease in blood flow to the nasal area which further dries secretions which can crust leading to a sense of nasal stuffiness (Sheahan and Musialowski, 2001).
In addition to the imbalance between the production of thick, tenacious mucus and thin watery mucus, the ageing process also affects muco-ciliary clearance, where the cilia lining the trachea and the smaller airways flatten, making it much more difficult to clear secretions from the lungs. There is also a decreased ability to cough up secretions as we age (Roach, 2001). In addition to having fewer mucous-producing cells, there is a decline in the normal bronchial secretion production which may cause epithelial damage and the risk of bacteria sticking to the bronchial walls (Dyer and Stockley, 2006).
The thoracic cage and muscular function
The costal cartilages, which connect the ribs to the sternum, the ribs to each other and also to the thoracic vertebrae become more rigid and stiff resulting in decreased compliance during quiet respiration (Roach, 2001). This change increases the need to use the accessory muscles for breathing. However, the strength in the respiratory muscles begins to decrease from about the age of 55 (Sheahan and Musialowski, 2001). The intercostal muscles that help move the chest wall atrophy and become weaker, so that the work of breathing is increased. As the intercostal muscles weaken and the ribcage becomes stiffer, the diaphragm plays a larger role in breathing, as it takes over a higher proportion of the mechanical effort needed for increasing ventilation (De Martinis and Timiras, 2003).
Dyspnoea on exertion is common among older people. Whether this is due to a decrease in muscle strength or an increase in thoracic stiffness and loss of lung compliance is unclear. However, it is evident that older adults’ respirations are shallower with increased diaphragmatic motion because of ageing changes in the costal structures, as described earlier when compared with younger adults (De Martinis and Timiras, 2003).
A number of changes occur with ageing in respiratory muscles (De Martinis and Timiras, 2003), namely:
- Muscle strength is decreased
- More prone to fatigue when the work of breathing is increased
- Atrophy of some respiratory muscles
- Ratio of anaerobic to aerobic metabolism is increased
- Blood supply to muscle is decreased.
Alveoli
With ageing, the structure of the alveoli also changes. The alveolar surface of a younger adult is ~70 m2 (Tortora and Derrickson, 2009). Sheahan and Musialowski (2001) suggest that this large surface area for gas exchange reduces with age. The alveoli themselves become shallower and flatten as a result of the loss of septal tissue (De Martinis and Timiras, 2003). The walls of the alveoli become thinner, and there is a decrease in the number of capillaries surrounding each alveolus. The alveolar ducts become stretched, resulting in the alveoli enlarging or tearing. However, the number of alveoli remains relatively constant. The changes in the structure of the alveoli can be attributed to changes in the elastic fibre network of the alveolar walls. There is also some evidence that the alveolar changes including the increased alveolar duct size, the reduction in surface area and decreased diffusing capacity could be related to the disruption of collagen fibres within them (Dyer and Stockley, 2006).
The pattern of air distribution alters with age, resulting in an increase in alveolar duct air but a decrease in alveolar air. From the age of 30, there is a decrease in alveolar air of 4% for each subsequent decade. As oxygen transport is most efficient in the alveoli, the decrease in alveolar air space will result in reducing optimal oxygen diffusion from alveolar air into pulmonary capillaries (De Martinis and Timiras, 2003). The reduction in oxygen movement from alveolus to pulmonary capillary may reduce overall oxygen concentration within the blood, thereby making the older adult more prone to hypoxia after little effort.
Elastic recoil
The lungs begin to distend as the elastic tissue stretches and elastic recoil decreases. Normal expiration is mostly a passive process that is attributable to recoil of the elastic tissue of the stretched lungs and thorax. However, the amount of elastic tissue decreases with the ageing process while the amount of fibrous tissue is increased. Not only does the amount of elastic tissue decrease, but also the alteration in its distribution has an effect on lung function, in that abnormal location or structure of the elastic fibres may contribute to impairment of ventilation and perfusion of the lungs. Collagen becomes more rigid and changes in its structure together with changes in elastin contribute to the loss of recoil.
Control of ventilation
The action of the carotid body and aortic body chemoreceptors appear to be less responsive to changes in blood oxygen and pH levels with advancing age (Roach, 2001). The respiratory centres in the brain located within the pons and the medulla may also be significantly less efficient in older people (De Martinis and Timiras, 2003). The ventilatory response to hypoxia is decreased by 51% in healthy men between the ages of 64 and 73 compared with healthy men between the ages of 22 and 30 (Roach, 2001). Older people are therefore less able to tolerate hypoxia or hypercapnia (Sheahan and Musialowski, 2001).
Changes in ventilation with ageing
Pulmonary ventilatory function steadily decreases after the age of 50. Changes in compliance within the respiratory system result in premature airway closure and poorly ventilated or unventilated alveoli at resting lung volumes. Small airways are more likely to collapse in the lung bases because of reduced elastic recoil. As a result of decreased elastic lung recoil, the bases of the lungs do not inflate well and secretions that collect in the lungs are not so easily expectorated.
Vital capacity, maximum ventilation rate and gas exchange all decrease with age. However, these effects are largely negated due to large reserve capacity in the respiratory system. Vital capacity decreases as the ability to fill and empty the lungs is reduced (weakening of respiratory muscles and stiffness of cartilage and ribs). As a result, maximum minute ventilation rates are reduced; this limits the person’s capacity for intense exercise. The decreased vital capacity of the lung that occurs with ageing is also thought to be associated with height and posture changes. While vital capacity decreases, the residual volume increases.
An increase in the diameter of the bronchioles and alveolar ducts leads to an increase in dead space which reduces the amount of air available for gas exchange. Partial alveolar wall collapse and thickening of the alveolar membrane further reduces gas exchange across the respiratory surface. However, oxygen requirements are generally less because of lower basal metabolism in older people (Dyer and Stockley, 2006). Blood gas levels of pO2 may fall slightly with ageing, but arterial pCO2 levels stay approximately the same.
With ageing, the respiratory system cannot accommodate the increased metabolic rate during exercise. Consequently, there is a greater increase in ventilation for a similar increase in workload compared to younger adults (Sheahan and Musialowski, 2001).
Exercise can delay the ageing changes associated with the mechanical properties of the lungs. Physical training can increase respiratory muscle function, maximal voluntary ventilation, maximal minute ventilation and static lung volumes. Ageing appears to affect lung tissue mechanics to a lesser degree than it does to chest wall dynamics. There is no evidence to suggest that habitual physical activity counteracts age-related changes in chest wall mechanics.
Assessing respiratory function
The nurse must be able to carry out a comprehensive respiratory assessment. Upon approaching the patient, the nurse should assess the rhythm, rate and depth of breathing. The patient’s colour should be noted, although on its own this is not a very reliable indicator of a patient’s respiratory status. Simple observation can also indicate use of accessory muscles of breathing or evidence of breathlessness (dyspnoea). The neck and back should be assessed for structural defects, such as deviation of the trachea, kyphosis or scoliosis – all of which interfere with, and limit, chest expansion.
The nurse should enquire whether the patient has had to limit their levels of activity or has been limited in their exercise tolerance due to breathing difficulties. The degree of breathlessness can be established by identifying the level of breathlessness:
1. Shortness of breath when hurrying on a level surface or when walking up hills or stairs
2. Shortness of breath when walking on a level surface with people of the same age
3. Shortness of breath when walking on a level surface at one’s own pace
4. Shortness of breath when washing or dressing
5. Shortness of breath when sitting quietly.
(Taken from Heath and Schofield, 1999, p. 126)
Another indicator of the extent of breathlessness is the distance the older person can walk on a flat surface without stopping to rest. Patients should also be asked if they have shortness of breath during the night or at specific times and also asked if breathlessness interferes with their lifestyle.
A smoking history should be taken including any attempts or desire to stop smoking. This should include a past and present history of smoking. The age that the person started smoking and the number of cigarettes smoked daily should be noted.
Any history of coughing should be identified, taking particular note of the time of day, and whether productive or not. Amount, if any, and type of phlegm should be noted. Also identify how long the cough has been present and the colour of the phlegm if present.
Exposure to pollutants should be acknowledged, and if people live in an industrial area where there are high emissions of environmental pollutants, this should be noted. Present and past occupations and hobbies may also indicate contact with possible respiratory irritants. Staff should also enquire if other factors such as cold, damp conditions or air conditioning aggravate the problem.
Common respiratory problems associated with ageing
In this section, we will examine a number of common conditions of the respiratory tract particularly associated with ageing.
Pneumonia
Pneumonia is a serious and life-threatening condition in older people. The death rate associated with pneumonia is estimated to be five times higher in older people over 65 when compared to people under the age of 65. In older populations, the condition usually requires a period of hospitalisation. Coexisting long-term illnesses may complicate the situation.
Pneumonia can be caused by a variety of factors including bacteria, viruses, fungi and aspiration of fluids (Mauk, 2006). It is an inflammation of the lungs usually caused by infection and is often associated with the alveoli filling with fluid, that is, to consolidate. This accumulation of exudate within the alveoli compromises gas exchange and can result in death. If the pneumonia has a patchy distribution, it is referred to as bronchopneumonia. Lobar pneumonia refers to a single segment or an entire lobe of lung affected. Older adults are particularly susceptible to this condition due to their reduced immune response associated with ageing changes, the possible existence of pre-existing respiratory illness, a reduced cough reflex and a reduction in levels of mobility (Meiner and Lueckenotte, 2006). The most common type affecting older adults is bacterial pneumonia (Roach, 2001).
Typical symptoms of bacterial pneumonia include cough, fever, sweating, shivering, loss of appetite and a feeling of being unwell. Headaches, and general aches and pains are also common. Sputum production increases and this may become yellow/green in colour. Occasionally, the sputum is bloodstained. The patient can become breathless, tachypnoeic and develop chest tightness. Pleuritic pain may develop which is often worse on inspiration.
However, pneumonia may manifest itself differently in older people when compared to younger people. Older people may exhibit increased respiratory rate and increased pulse rate as the first symptom with respirations consistently over 26–28 per minute. Additional symptoms include a general deterioration and changes in mental status. Typical symptoms of cough, chest pain, production of sputum and fever are not always present, making pneumonia difficult to detect in older patients (Roach, 2001). Older adults may also demonstrate symptoms of dehydration and confusion (Meiner and Lueckenotte, 2006).
Viral pneumonia symptoms are initially similar to influenza symptoms, with fever, a dry cough, headache, muscular pain and a generalised weakness. Within 24 hours, there is increasing breathlessness, a worsening cough and a small amount of mucus may be produced. Pyrexia is common. Viral pneumonias may be complicated by secondary bacterial invasion, leading to a picture similar to that seen in bacterial pneumonia.
Diagnosis is confirmed with chest X-ray, sputum culture and full blood count. Clinical history and examination should always be obtained. Auscultation may demonstrate ‘crackles’ in the lungs.
Viral pneumonia generally clears by itself, but bacterial pneumonia will require appropriate antibiotic therapy. The causative agent should be identified by obtaining a sputum sample and sending it for culture and sensitivity to determine the antibiotic of choice. The duration of therapy is also dictated by the nature of infection and responses to treatment; however, antibiotic therapy is generally administered for 10–14 days as required (Meiner and Lueckenotte, 2006). Adequate levels of hydration (1500–2000 ml/day) should be maintained, nutritional status assessed and adequate nutrition given. Sufficient rest is also helpful. Complications following respiratory illness often lead to death in older people (Mauk, 2006). Older adults should therefore be reminded to contact their health-care professional if their respiratory status does not improve.
The older adult should consider the annual influenza vaccination as pneumonia is often a complication of influenza. A one-off vaccine against pneumonia is also available for them.
Management of the older adult with pneumonia
Vital signs (temperature, pulse, blood pressure and respirations) should be monitored as frequently as the patient’s condition dictates. Any increase in respiratory and/or pulse rate should be reported to senior staff. Staff should also examine the patient’s nail beds, lips and oral mucosa for evidence of cyanosis and record their findings. Sputum should be monitored for amount, colour, consistency and presence of blood.
Regular pain assessment is required to identify and treat any pleuritic pain or pain associated with coughing. Analgesia should be administered as prescribed and evaluated for effectiveness.
Patients should also be assessed for restlessness, confusion and drowsiness as they may be an indication of decreased oxygenation of the brain. Oxygen saturation should be monitored closely. A reduction of oxygenated blood flow to the brain is closely linked to delirium.
Patients with pneumonia may report fatigue, decreased activity tolerance and loss of appetite. Activities should therefore be planned to alternate with rest periods in order to prevent exhaustion. Assistance with activities such as personal hygiene needs and personal care will also reduce the risk of fatigue. Meals offered should be nutritious, small and frequent with assistance being offered as required. A quiet environment will do much to promote rest and sleep.
It may be necessary to administer supplemental oxygen to an older adult with pneumonia in order to correct dyspnoea and hypoxia. Oxygen is a gas that is present in air at a concentration of 21%. Pharmacologically, oxygen is a prescription only medication under the Medicines Act 1968, and therefore, except in an emergency, oxygen should always be prescribed by a medical practitioner.
Oxygen can be delivered in a variety of ways. The most usual means of administering oxygen are by facemask or nasal cannulae. Nasal cannulae have the advantage of allowing the person to eat, drink and speak without interrupting the delivery of oxygen (Jevon and Ewens, 2001).
When administering oxygen, safety issues are of paramount concern. The nurse should promote and ensure patient safety during the administration of oxygen and adhere to local policies, guidelines and national protocols as appropriate (Francis, 2006). Oxygen will support combustion and therefore must not be used near a naked flame or sources of static electricity. White soft paraffin or other oil-based products or face creams should not be used to relieve dryness or soreness to the nose as these products are potentially combustible. Furthermore, these have a tendency to clog nasal cannulae. It is important to reassess the patient frequently to confirm that they continue to receive the prescribed dose of oxygen and that the mask or cannulae remain in position and comfortable (Jevon and Ewens, 2001).
To avoid the drying effects of oxygen, humidification should be used whenever possible and the nurse should ensure a good fit of the mask or nasal cannulae. The patient should be encouraged to drink between 1500 and 2000 ml of fluids to maintain good hydration and to help loosen bronchial secretions (Roach, 2001). Oral hygiene should be offered to the patient receiving oxygen as they may experience drying of the mouth.
It is important that the nurse establishes a good rapport with the patient in order to offer support and reassurance regarding their oxygen therapy. Additionally, the nurse should ensure that the patient has a good understanding of the reasons for the prescription of oxygen. Where appropriate, this should also include relatives and carers (Jevon and Ewens, 2001). This will help maintain patient compliance with this potentially demanding therapy.
 Point for Practice
Point for Practice
Hamish, aged 78, is due to be discharged home and to continue oxygen therapy at home. Outline a plan of care to prepare Hamish and his family for this therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





