T score
Normala
At or over −1,0 SD
Osteopenia (low bone mass)b
Ranges between −1.0 and −2.5 SD
Osteoporosisc
At or below −2.5 SD
Severe or established osteoporosis
The existence of one or more fractures due to a mild trauma (such as a fall while standing up) in addition to a T score below −2.5 SD
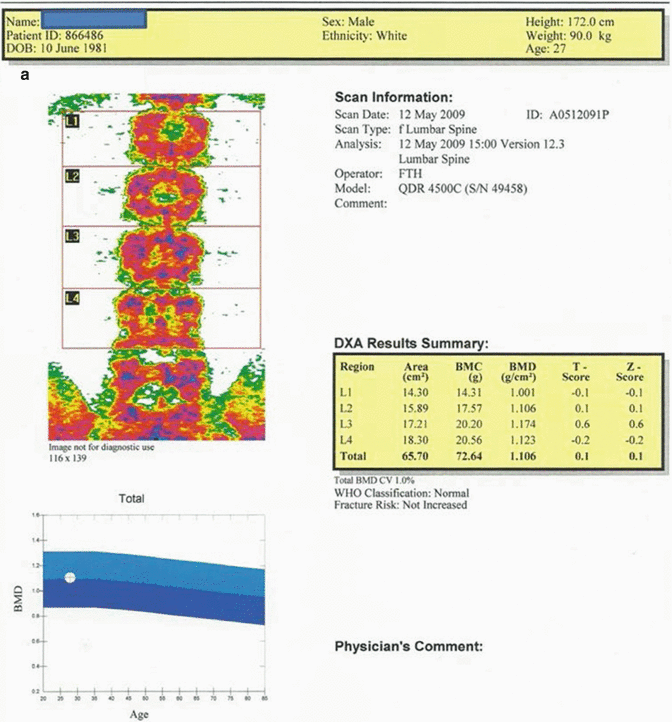
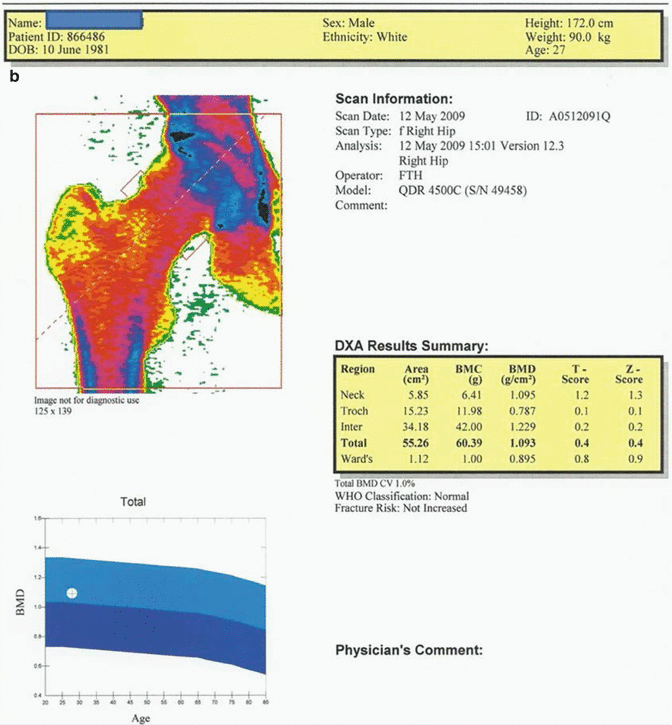
Fig. 12.1
(a) 27-year-old male patient suffering from hypogonadism. Average T and Z scores of L1–L4 vertebrae are calculated to be 0.1 and 0.1, respectively. The fracture risk is not increased, and Z score is to be evaluated as “within the expected range for this age” since the case is a male patient below 50 years of age. (b) The average T score for proximal femur in the same case is 0.4; Z score is 0.4. Fracture risk is not increased, and Z score is to be described as “within the expected range for this age” since the case is a male patient below 50 years of age
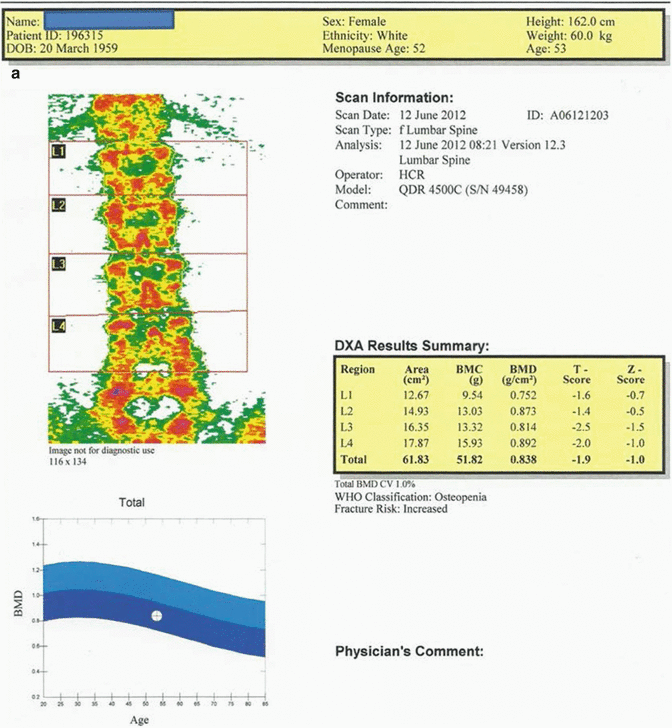
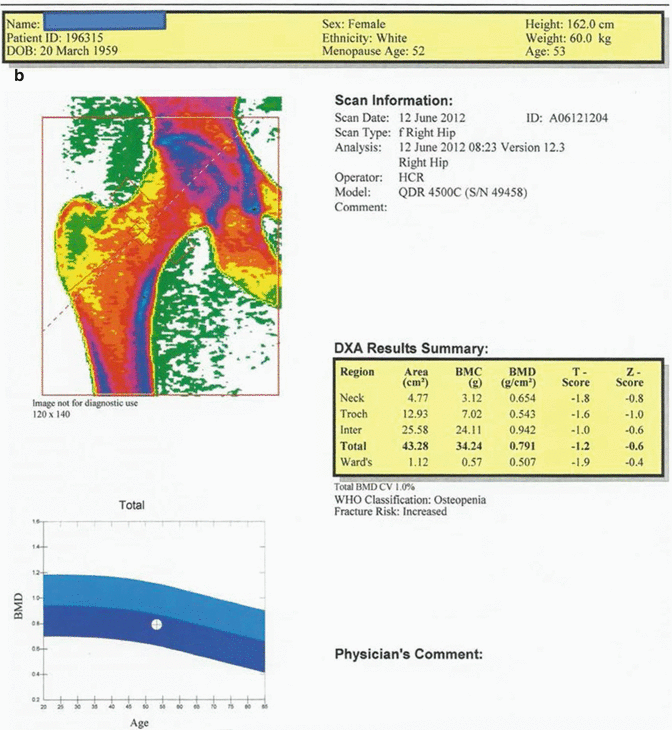
Fig. 12.2
(a) 53-year-old female patient. The average T score of L1–L4 vertebrae is −1.9, and Z score is −1.0. The BMD value is consistent with “osteopenia” range by WHO criteria and the fracture risk is increased. (b) The average T score for proximal femur in the same case is −1.2; Z score is −0.6. Although BMD value is consistent with “osteopenia” by WHO criteria, the fracture risk is increased
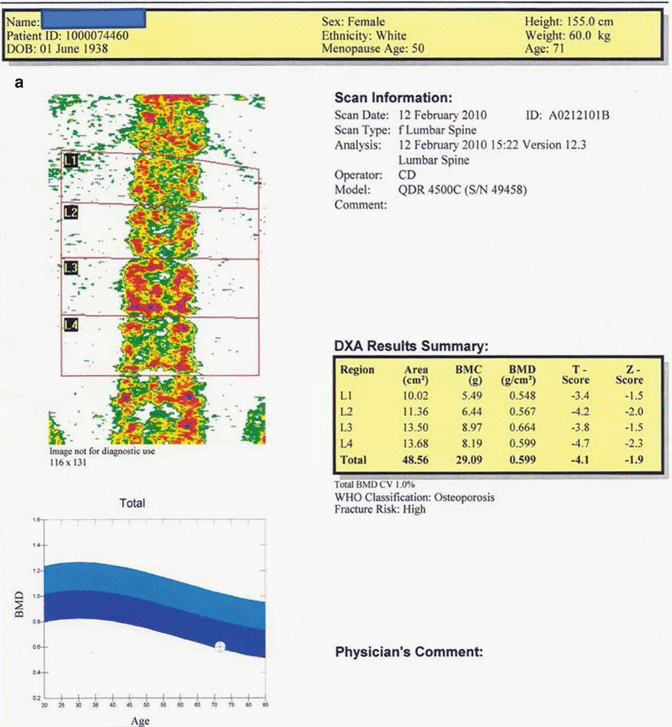
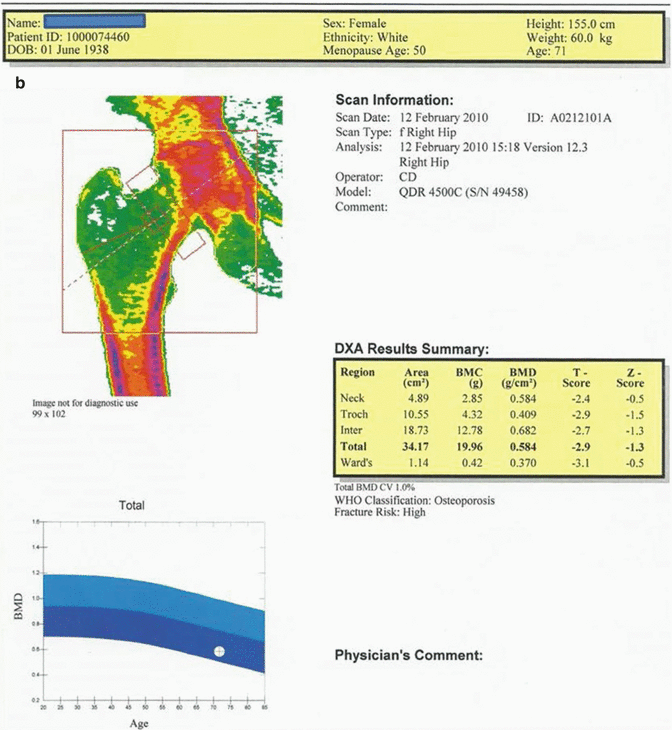
Fig. 12.3
(a) 71-year-old female patient. The average T and Z scores of L1–L4 vertebrae are −4.1 and −1.9, respectively. The BMD value of this case is consistent with “osteoporosis” by WHO criteria and the fracture risk is high. (b) The average T and Z scores for the proximal femur in the same case are −2.9 and −1.3, respectively. The BMD value is consistent with “osteoporosis” by WHO criteria, and the fracture risk is high
T score should be used for the diagnosis of osteoporosis in postmenopausal women and men over 50 years of age.
Z score should be used in premenopausal women, men under 50 years of age, and children.
The International Society for Clinical Densitometry (ISCD) states that:
Z score under −2 SD should be defined as “low bone mineral density below the expected range for chronological age.”
Z score over −2 SD should be defined as “bone mineral density within the expected range for chronological age.”
Z score is also especially important for the patients aged 75 years and over. ISCD also states that these populations cannot be diagnosed with osteoporosis only by using dual-energy X-ray absorptiometry (DXA) [1, 5, 8].
The proximal femur is not a reliable measurement location in pediatric patient group because of the variations in the development of skeletal system and a low level of repeatability in locating the field of interest. Lumbar vertebras (PA) and whole-body measurement excluding the head are the recommended locations for BMD measurement in children. Z score should be used instead of T score in the reports of pediatric patients. Besides that, the term “osteopenia” and, as long as there is no clinical history of fracture, the term “osteoporosis” should not be used in the pediatric patient group [9].
Patients with either osteoporosis or under the risk of developing fractures constitute the most important patient group for BMD measurement. BMD results must be comparatively evaluated in terms of age and sex for a more precise assessment. The fracture threshold for any individual is defined as 2.5 standard deviation (SD) below the BMD value of a young adult. While the fracture risk is almost nonexisting in an osteopenic patient with a T value of −1.1 SD, it is almost as much as an osteoporotic patient’s risk in a person with a T value of −2.4 SD [2]. On the other hand, every unit of decrease in standard deviation increases the risk of femoral fracture 1.5–2 times [1, 5]. In addition to a low BMD value, the existence of femoral fracture or osteoporosis in the family, a low level of bone mass, use of steroids, smoking and alcohol habits, low calcium and vitamin D intake, and environmental factors increasing the risk of falling are defined as the risk factors for fractures [2]. Although the loss of BMD is related to the whole skeleton, it is more explicit in bones with higher trabecular density such as vertebras, femoral neck (Ward’s triangle), and distal radius. BMD does not range in the same interval for the whole lifetime. It decreases in parallel with aging after the young adult age period. For example, BMD in the femoral neck decreases about 0.3 % every year in the third to fifth decades of a person’s lifetime which means that a person’s BMD value may decrease below the osteoporotic level due to age-associated bone loss even with no additional reason [2].
The main target in the treatment of low BMD is to get increase in bone mass to prevent fractures. Calcium and vitamin D should be taken both with diet and from sources external to diet. For the treatment of patients at the age of 80 and over, 1,500 mg calcium and 800 units of vitamin D intake is advised on a daily basis [2]. Bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) are also suggested in order to limit bone resorption and increase bone mass [2].
BMD measurement is advised for all menopausal women at the age of 65 and over and for all men over 70 years old regardless of risk factors, as well as for menopausal women aged below 65 years old with at least one risk factor, for premenopausal women over 40 years old with at least one history of a fracture associated with light trauma, and for all patients on osteoporosis treatment and on long-term steroid use [2]. In young people and in children, BMD should be measured if causes for secondary osteoporosis exist [9]. Main indications for BMD measurement are summarized in Table 12.2 [8, 10–13].
Table 12.2
Main indications in BMD measurement
Women aged 65 and older and men aged 70 and older, regardless of clinical risk factors |
Younger postmenopausal women and men aged 50–69 with clinical risk factors listed below for fracture |
Existence of osteopenia and/or vertebral pathology in radiology |
History of fragility fracture (hip, vertebra, radius, proximal humerus fractures) |
Smoking and increased alcohol consumption |
History of osteoporotic fracture in family |
Existence of significant shortening and thoracic kyphosis |
Premature menopause (<45) |
Prolonged secondary amenorrhea |
Primary hypogonadism |
Corticosteroid treatment (>5 mg/day or equivalent for ≥3 months) |
Anorexia nervosa and malabsorption |
Primary hyperparathyroidism |
Osteogenesis imperfecta |
Hyperthyroidism |
Long-term immobility |
Cushing syndrome |
Neoplasia (multiple myeloma and others) |
Follow-up of treatment efficiency in patients being treated for osteoporosis |
Cases that are not being treated but with possibility of bone loss that would require treatment |
Women and men before 50 years of age with causes of secondary osteoporosis |
BMD Measurement Methods
The method of BMD measurement should be easy, cheap, reproducible, and specific, and it should provide sufficient and accurate information on the risk of fracture in advance. Other qualifications desired in the method are a low dose of radiation and comparability of the test to the standards of race, age, sex, and region in which the test is performed, providing sufficient and fast information for treatment follow-up and compatibility with other diagnostic tests (biochemical tests, biopsy, etc.) used for osteoporosis [14].
We will now examine briefly today’s most commonly used BMD measurement methods listed in Table 12.3.
Table 12.3
Commonly used BMD measurement methods
General classification | Method |
|---|---|
Radiological methods: | Standard conventional radiography |
Bone radiometry | |
Radiologic photodensitometry | |
Digital image processing (DIP) | |
Quantitative computed tomography (QCT) | |
Photon absorption techniques: | Single-photon absorptiometry (SPA) |
Dual-photon absorptiometry (DPA) | |
Single-energy X-ray absorptiometry | |
Dual X-ray absorptiometry (DXA) | |
Other methods: | Quantitative ultrasonography (QUS) |
Neuron activation analysis | |
Magnetic resonance imaging | |
Slit screening flography | |
Bone biopsy |
Quantitative Computed Tomography (QCT)
Computed tomography (CT) measures BMD in Hounsfield units as mg/cm3 using single or dual energy [6]. One of the most important problems in QCT is that bone mineral content can be measured 15–20 % lower than its real value because of the increasing fat content in the bone marrow by age. Use of dual-energy technique provides more accurate results due to the capacity to correct the effect of fat in the bone marrow. Besides, this error associated with fat content is accepted as clinically insignificant since age is used to determine the risk of fracture [6].
It is important to be able to measure the BMD value of trabecular bone separately in patients with osteoporosis, since it is more sensitive in monitoring the changes in the course of disease and evaluation of response to the treatment [1, 5]. The overall sensitivity of QCT as an effective method in the estimation of vertebral fractures and in the measurement of bone loss with the capability of measuring trabecular and cortical bone density separately is higher than the overall sensitivity of DXA. The front part of the trabecular bone in vertebral corpus is used for analysis with QCT. It is also possible to measure trabecular bone selectively by excluding concentrations that can lead to a higher BMD value inaccurately, such as aortic calcification in the field of measurement [1]. Despite all these advantages, QCT is not often used in BMD measurement because of its high cost and higher radiation exposure (1.5–2.9 mSv) compared to other methods [5].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








