Fig. 11.1
Arthroscopic image demonstrating near full-thickness cartilage loss on the lateral femoral condyle in the knee of a patient with lateral meniscus deficiency. In many cases, meniscus damage is the main factor in cartilage degeneration due to increased stresses on the articular cartilage
Healing rates following meniscal repair vary based upon the location of the tear within the knee (medial vs. lateral), location within the meniscus itself (central vs. peripheral), and whether a concomitant anterior cruciate ligament (ACL) reconstruction is being performed. Lateral compartment and peripheral tears with less than 3 mm of rim width are more likely to heal [4]. Successful repairs have been reported to be anywhere from 74 to 96 % when anterior cruciate ligament (ACL) reconstruction is undertaken in the acute injury setting [5, 6]. For isolated meniscal repairs without ACL reconstruction, however, healing rates are less predictable [7–9]. Most authors speculate the reason for this improved healing following ACL reconstruction is the local delivery of growth and clotting factors from the intra-articular hematoma that develops following femoral and tibial tunnel drilling. In addition, the nature of meniscal injury may differ in the setting of concomitant ACL injury, and meniscal repair may be more successful in this group due to more favorable anatomic tear characteristics. In other words, meniscal tears in conjunction with ACL injury tend to occur more peripherally and usually involve less degenerative tissue.
Given the observation of improved healing rates following concomitant ACL reconstruction and the role of adjunctive growth factors, much attention has been paid to the biologic enhancement of meniscus repairs. Areas of focus have centered on the use of growth factors and cell-based therapies. When repair is not possible, meniscal replacement may be considered. This chapter will review the biology of meniscus healing and use this as a foundation to discuss the enhancement of meniscus repair through mechanical stimulation, growth factors, and gene therapy. It will also discuss current options for meniscal replacement in the meniscus-deficient knee.
Biology of Meniscus Healing
Healing of meniscal repairs is mainly dependent upon tear location as only the outer 10–25 % of the meniscus receives direct blood supply from the perimeniscal capillary plexus (PCP) [10]. This plexus is supplied by the lateral, medial, and middle geniculate arteries and originates in the capsular and synovial lining of the joint. Nutrient and waste exchange for cells in the inner portion of the meniscus relies on diffusion through the synovial fluid [11]. Blood supply is critical for healing success as the presence of growth and clotting factors simulates the repair process [12–14]. It has been shown that when exposed to these factors, particularly platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and fibronectin, meniscus fibrochondrocytes are stimulated to proliferate and produce extracellular matrix [15–17].
When a tear occurs in the peripheral portion of the meniscus, a fibrin clot forms which contains inflammatory cells. The PCP then infiltrates the fibrin clot, delivering stem cells and growth factors to aid in the differentiation and growth of the fibrochondrocytes [18, 19]. Some of the growth factors that have been implicated in meniscus healing are PDGF, TGF-β, insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) [19–21]. In an investigation comparing the effect of bFGF, IGF-I, PDGF, and TGF-β on protein and proteoglycan production in bovine meniscus tissue explants, Imler et al. found that all four increased cellular production, with TGF-β being the most potent stimulator [22]. Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1), on the other hand, have been shown to have a deleterious effect on fibrochondrocyte proliferation in vitro [23]. The expression of other molecules, such as the antiangiogenic factor endostatin, also may limit the regeneration capacity of the meniscus [24].
Mechanical Stimulation
Mechanically stimulating the damaged tissue provides an injury response, potentiates vascularity, and is one of the simplest ways to favor healing of the meniscus. The most common techniques utilized are rasping (Fig. 11.2) and trephination. These methods create radially oriented channels to theoretically induce vascular and cellular migration from the periphery to the repair site. Ochi et al. studied the effect of rasping on the presence of TGF-β, PDGF, interleukin-1-alpha (IL-1α), and proliferating cell nuclear antigen (PCNA) on the femoral surface of the menisci following rasping [17]. They found that levels of IL-1α, TGF-β, PDGF, and PCNA on the rasped surface area all reached their peak within 14 days after surgery. Many studies have reported improved healing rates of meniscus tears treated with mechanical stimulation as compared to without [25–27].
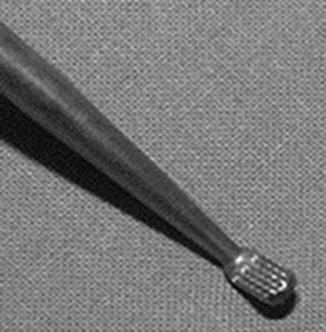

Fig. 11.2
Image of an arthroscopic rasp used for mechanical stimulation of tissue prior to meniscal repair. The rasp is inserted into the meniscal tear in order to improve vascularity at the repair site
Fibrin Clot
Exogenous fibrin clot was one of the first substances studied to aid in the healing of meniscal tears. In 1985 Weber et al. demonstrated that rabbit meniscus fibrochondrocytes proliferate and produce matrix proteins when exposed to the mitogenic factors contained in a wound hematoma [15]. Based upon this initial work, Arnoczky et al. studied the effect of exogenous fibrin clot application on the healing rate of meniscal tears in the avascular region of canines [10]. At 3 and 6 months postoperatively, they reported that all the defects had filled with fibrocartilage-like material. Clinical case series utilizing fibrin clot followed, with van Trommel et al. reporting healing on second look arthroscopy in all patients following repair of peripheral posterior-lateral meniscus tears supplemented with fibrin clot [28]. In a series of 153 meniscus tears with or without concomitant ACL tear, Henning et al. reported a healing rate of 92 % when treated with exogenous fibrin clot versus 59 % for those treated without fibrin, concluding that isolated meniscus tears heal significantly better with the addition of the exogenous agent [29]. In a randomized trial comparing conservative therapy, arthroscopic suture repair with access channels, arthroscopic minimal central resection with intrameniscal fibrin clot and suture repair, and arthroscopic partial meniscectomy, Beidert found 75 %, 90 %, 43 %, and 100 %, respectively, of normal or nearly normal exam and MRI at final follow-up [30]. In other words, the group receiving meniscus repair and fibrin clot application had the lowest clinical and imaging scores. Each group in this study, however, included 12 or fewer patients (with the fibrin clot group consisting of only seven patients), potentially limiting the applicability of this investigation to the larger population.
Platelet-Rich Plasma
Platelet-rich plasma (PRP) is an autologous substance that contains concentrations of platelets above physiologic levels (Fig. 11.3). These platelets are a source of PDGF, TGF-β, IGF-1, VEGF, and bFGF, among others, and are thought to initiate a healing cascade leading to angiogenesis, matrix production, and cellular proliferation [31–33].


Fig. 11.3
Image showing a syringe with whole blood components following centrifugation. The three layers include the red blood cell base (bottom), the buffy coat consisting of platelets and leukocytes (middle), and acellular plasma (top)
Ishida et al. performed the most comprehensive basic science and animal investigations to date on the effect of PRP on meniscus healing [34]. Using a gelatin hydrogel (GH) to deliver PRP to rabbit meniscal fibrochondrocytes in vitro, they found that levels of growth factors (PDGF, TGF-β, and VEGF) as well as messenger RNA (mRNA) for biglycan and decorin were all higher in cultures supplemented with PRP as compared to platelet-poor plasma (PPP). The authors then used the same delivery system to treat 1.5 mm diameter defects in the avascular zone of rabbit menisci. At 4 weeks, they found residual presence of the hydrogel, indicating elution of the growth factors from PRP took place over this timespan. Histological scoring of the menisci at 12 weeks showed significantly better meniscal healing in the group treated with PRP-infused hydrogel as compared to the hydrogel with PPP or the hydrogel alone [34].
Another investigation examined the effect of hyaluronan-collagen composite matrices without cells, the composite matrices loaded with platelet-rich plasma, autologous bone marrow, or autologous mesenchymal stem cells (MSCs) to treat 2 mm punch defects in the avascular zone of rabbit menisci [35]. At 12 weeks, the authors reported the untreated defects and cell-free composite matrix groups both had a limited fibrocartilaginous healing response. Interestingly, they found that neither the PRP nor bone marrow-loaded matrices showed histological grading improvement as compared to cell-free implants.
Stem Cells
Under the proper conditions, MSCs have the capability of differentiating into a number of different cell lineages and therefore represent a promising option in the augmentation of meniscus repair. There has been documentation of MSCs availability from bone marrow [36] (Fig. 11.4), periosteum [37], adipose tissue [38], and the synovial lining of major joints [39]. In both cartilage and meniscus restoration investigations, they are commonly delivered to the desired treatment area through the use of a cellular scaffold [40, 41].
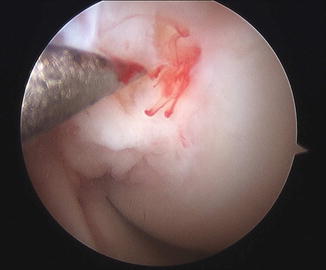

Fig. 11.4
Arthroscopic image showing microfracture of the wall of medial femoral condyle within the notch. This technique allows for mesenchymal stem cells (MSC) within the bone marrow to have access to the meniscus itself and augment healing
Bone Marrow-Derived Stem Cells
Using autologous-derived bone marrow MSCs in a rabbit meniscus defect model, Zellner et al. studied the effect of a precultured chondrogenic MSC matrix construct versus non-precultured stem cell-matrices [35]. Preculturing of the MSCs took place in a chondrogenic medium and MSCs were implanted in a hyaluronan-ester and gelatin scaffold. They found that the precultured cells lead to fibrocartilage-like repair tissue and had only partial integration with the native meniscus. On the other hand, non-precultured MSC matrices demonstrated more complete fill and meniscus-like healing on histological sectioning [35]. Other investigations utilizing different animal models and a variety of scaffolds have shown promising results with the use of bone marrow-derived MSCs [42–44].
Yet another way to allow MSC delivery to the meniscus in order to augment healing is with the use of microfracture [45]. In their article, Freedman et al. describe the use of microfracture following standard techniques for mechanical stimulation and then inside-out meniscus repair, although the microfracture technique can be utilized following any meniscus repair method. Following repair, a 45° microfracture awl is placed through the contralateral portal. The awl is repeatedly penetrated through the subchondral bone of the intercondylar notch at the posterior cruciate ligament (PCL) origin until marrow elements are seen to enter the joint. The flow of arthroscopic fluid can be halted to more accurately confirm blood flow emanating from the microfracture sites [45].
Synovial-Derived Stem Cells
Many investigators advocate for the use of synovial-derived MSCs as they can be easily harvested and have shown the ability to easily expand in culture [46]. Using autologous MSCs harvested from rabbits, Horie et al. studied regenerative efforts after cylindrical defects were created in the avascular portion of the medial meniscus [47]. During the index procedure, the defect was created through an open arthrotomy in each knee. One knee received a direct injection of suspended MSCs, while the other received only an injection of phosphate buffered saline (PBS) as a control. They found that the quantity of the regenerative tissue was greater at all timepoints for the MSC group, reaching significance at the 4- and 12-week marks, while the quality of the regenerative was also significantly higher at 12 and 24 weeks. Another investigation which injected synovial-derived MSCs into the knees of meniscectomized rats found improved adherence to the defect site, differentiation into meniscal cells, and promotion of meniscus regeneration in the MSC group as compared to controls [47].
Adipose-Derived Stem Cells (ADSC)
Yet another source of MSCs is from adipose tissue. Ruiz-Iban used ADSCs to investigate meniscus healing potentiation in a rabbit model [48]. Using the contralateral knee as a control, they injected labeled MSCs directly into the tear after either immediately repairing the lesion with suture or after performing the repair 3 weeks later. Results showed that the addition of ADSCs to the repair site significantly increased the healing rate with the repair sites showing well-formed meniscal fibrocartilage made up of cells which had differentiated from the previously injected ADSCs.
Meniscus Replacement
Meniscus repair is not always possible, particularly in the case of complex tears. Debridement of large tears may lead to meniscal deficiency after debridement. In young patients who are not candidates for arthroplasty, meniscus replacement is an option. Choices for replacement include allograft transplantation, collagen-based scaffolds, and synthetic scaffolds.
Allograft Transplantation
In contrast to collagen-based and synthetic scaffolds, meniscus allograft transplantation can be used in cases of complete meniscus deficiency (Fig. 11.5a, b). The procedure has shown good results at mid- and long-term follow-up. In a study of patients undergoing both isolated transplant and combined procedures, Saltzman et al. reported significant improvement in all functional scores as well as an average overall satisfaction of 8.8 out of 10 [49]. Overall success rate at a minimum of 7-year follow-up was 88 %. Another investigation of meniscus transplantation with or without high tibial osteotomy (HTO) with a minimum 10-year follow-up reported a significantly improved modified Hospital for Special Surgery (HSS) score for all groups [50]. Those undergoing combined medial meniscus transplantation (MMT) and HTO showed greater functional improvement compared to MMT alone. There was no progression of radiographic changes in 41 % of knees and no further changes in cartilage signal on MRI in 36 %. In combination with articular cartilage repair or restoration, meniscus transplantation only has a reported 12 % failure rate [51]. Rue et al. reported on patients undergoing meniscus transplantation in combination with either autologous chondrocyte implantation (ACI) or osteochondral allograft (OA). At a minimum of 2-year follow-up, 76 % of participants reported satisfaction with their clinical results and 90 % of participants would have the surgery again [52]. A number of other studies have supported the use of transplantation for those with meniscus deficiency [53–55].
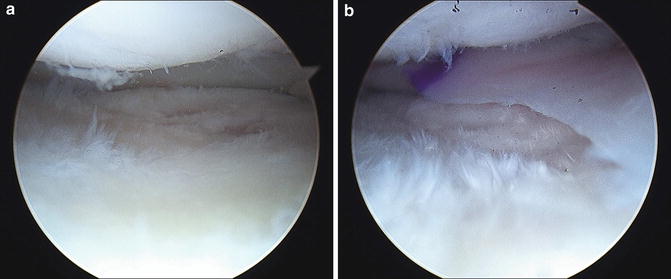

Fig. 11.5
(a) Arthroscopic image showing a near complete meniscectomized state in the medial compartment of a 24-year-old female. (b) Same compartment following meniscus transplantation with a size-matched allograft using bone block fixation
Collagen-Based and Synthetic Menisci
Unlike allograft meniscus transplantation, use of collagen or synthetically manufactured menisci requires an intact anterior and posterior horn as well as a small peripheral rim where the meniscus can be attached. Manaflex® or collagen meniscus implant/CMI (ReGen Biologics, Hackensack, NJ, USA) is an artificial meniscus derived from bovine collagen which is not currently approved for use in the United States. An initial report on eight patients showed promising results at short-term follow-up [56]. This was followed by a multicenter, randomized clinical trial comparing the use of the implant versus meniscectomy in over 300 patients with acute and chronic meniscal deficiency [57]. With a mean duration of follow-up of 59 months, the authors reported that in the chronic group, patients that had received the implant regained more of their lost ability as compared to the control meniscectomy group. There were no statistically significant differences in functional outcome in the acute replacement group.
Synthetic meniscus scaffolds have also been investigated. Actifit® (Orteq Bioengineering, London, UK) is a biodegradable polyurethane scaffold designed for meniscus replacement. In a recently published case series, 52 patients with irreparable meniscal defects were implanted with the synthetic scaffold [58]. Statistically significant improvement in International Knee Documentation Committee (IKDC), Knee Injury and Osteoarthritis Outcome Score (KOOS), and Lysholm scores was seen at 2-year follow-up as compared to preoperatively. Overall treatment failure rate was 17 % and there were nine implant related serious adverse events requiring reoperation.
Enzymatic Inhibition
Increased levels of inflammatory cytokines are associated with injuries to the knee. These inflammatory cytokines increase enzymatic tissue degradation and suppress matrix biosynthesis [59]. Investigations have demonstrated that IL-1 dose dependently decreases the shear strength of meniscus repair through inhibition of tissue formation at the meniscus repair interface [60–62]. Hennerbichler et al. harvested cylindrical explants from the outer one-third of the medial meniscus in a porcine model. The explants were immediately replaced and the entire specimen was incubated in medium with and without recombinant porcine IL-1. Those cultured in medium with IL-1 showed no discernable repair tissue despite the presence of viable cells [62]. The mechanism through which IL-1 provides its detrimental effects to meniscus healing is not fully understood. Recent evidence, however, has shown that matrix metalloproteinases (MMP) may play a role. McNulty et al. demonstrated that even in the presence of IL-1, a broad-spectrum MMP inhibitor increased the shear strength of meniscus repair sites and enhanced tissue repair at the interface compared to samples without MMP inhibitor [63]. Given these data, the search is underway for the optimal cytokine and/or MMP inhibitor in the attempt to enhance meniscus healing rates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








