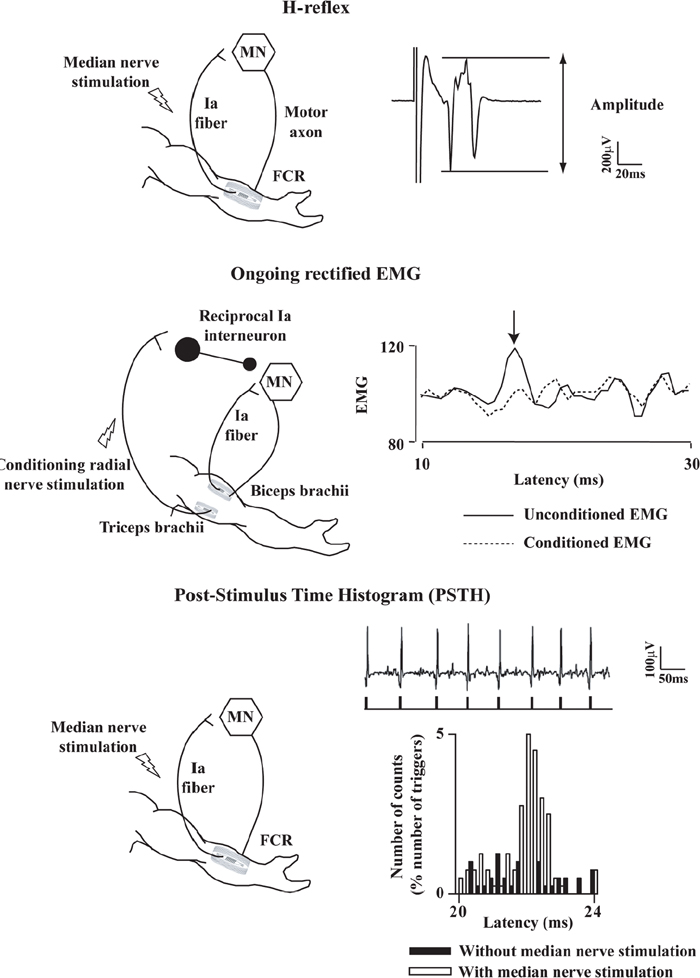
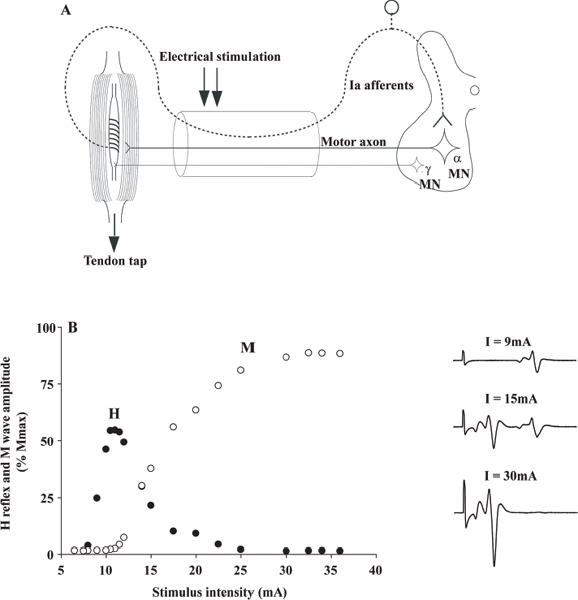
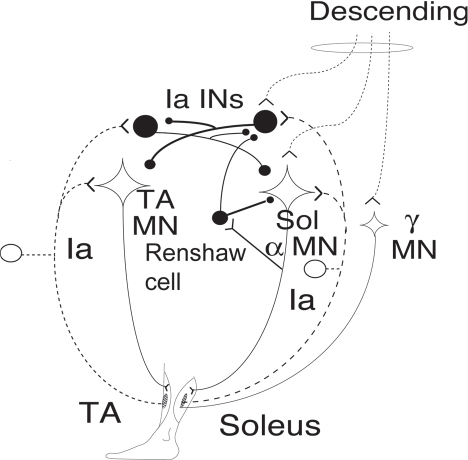
40 Key Points 1. Monosynaptic reflexes, ongoing EMG modulation, and post-stimulus time histogram are indirect techniques that allow probing of spinal circuitries in humans. 2. Recurrent inhibition of motoneurons, reciprocal to inhibition, and presynaptic mechanisms are described in healthy subjects and spinal cord injury (SCI) patients, both at rest and during motor activities. Noninvasive studies of central nervous system (CNS) networks in humans have been considerably improved by the progress in imaging techniques, including functional magnetic resonance imaging (fMRI), positron emission tomographic (PET) scanning, magnetoencephalography (MEG), and electroencephalography (EEG). However, up to now, these techniques have been suitable mainly for probing cerebral activities. At the spinal cord level, the small size of networks and motoneuron (MN) pools prevents their functional study with imaging techniques. Fortunately, the activity of muscle fibers is easily recorded with electrodes placed on the skin over a muscle belly. Because muscle fiber activity (except in rare pathological cases) relies only on MN activity, EMG is a “window” through which to explore MN excitability (Fig. 40.1). The knowledge of motor control in animals has emerged from experiments performed with monosynaptic reflexes (i.e., with techniques whose underlying principles are similar to those currently used in humans). Their validities have been fully confirmed with direct recordings from MNs. Even though methods used in humans cannot be controlled as those in animals, congruent results obtained with methods relying on different principles as well as control experiments performed in cats ensure their reliability. Moreover, it is likely that the effects of therapeutics aimed at facilitating CNS plasticity or regeneration in spinal cord injured (SCI) patients will first be detected with electrophysiological techniques, with clinical or MRI modifications being delayed. The pathway of the monosynaptic reflex arc is sketched in Fig. 40.2A: Ia afferents from muscle-spindle primary endings have monosynaptic connections to a MN innervating the muscle from which Ia afferents originate. This pathway underlies both the Hoffmann reflex (H-reflex) and the tendon jerk (T-reflex). In humans, at rest, percutaneous electrical stimulation of peripheral nerves usually evokes H-reflexes in the soleus, quadriceps, and flexor carpi radialis (FCR) muscles and, to a lesser extent, in hamstrings. A typical example of soleus H-reflex recordings and recruitment curves is illustrated in Fig. 40.2: when the intensity of the electrical stimulus is progressively augmented, a monosynaptic response (∼ 30 msec latency) appears on electromyography (EMG) and gradually increases in amplitude. When the threshold for motor activation is reached, a direct motor response (∼ 10 msec latency) appears on EMG, increases with the intensity of the electrical stimulus before reaching a maximum: the maximal motor response (Mmax) reflecting the discharge of the totality of α motor axons contained in the nerve. In proximal muscles (i.e., biceps or triceps brachii), motor and reflex responses are evoked together and overlapped. The T-reflex can also be used to assess the excitability of the MN pool via monosynaptic reflex amplitude: transient mechanical tap, elicited by an electromagnetic hammer, results in a tendon jerk in a wide range of muscles. However, the mechanical percussion is not as stable as the electrical shock applied on the peripheral nerve. Both H- and T-reflexes are simple, painless, and do not require the cooperation of the subject, only that the subject remain quiet. An excitatory input to the MN pool (i.e., induced by a conditioning stimulation) will increase the amplitude of unconditioned monosynaptic reflexes, whereas an inhibitory input will suppress it. There are some limitations to the use of changes in monosynaptic reflex amplitude to assess changes in MN excitability: (1) monosynaptic reflexes are mediated by Ia afferents, so the mechanisms controlling the Ia afferent volleys, including presynaptic Ia inhibition and postactivation depression (see later discussion), have to be taken into consideration; and (2) there are some limitations due to both properties of the MN pool and inhomogeneous distribution of afferent inputs within the MN pool.1 On the whole, the H-reflex technique remains the most valuable method for investigating spinal pathways in healthy subjects and patients with CNS lesions, whether at rest or during motor activities, when the following rules are taken into account: (1) the amplitude of the unconditioned H-reflex amplitude must be within the ascending part of the recruitment curve (Fig. 40.2); (2) the H-reflex size has to be expressed as a percentage of the Mmax; (3) the unconditioned H-reflex size has to be the same in all the situations (rest, motor activities, etc.); and (4) conditioned and unconditioned reflexes have to be randomly alternated. In this method, the ongoing EMG is rectified to sum both positive and negative deflections in the raw EMG and is therefore averaged. The ongoing unconditioned EMG is compared with that following a conditioning stimulus (conditioned EMG). An excitatory input to the MN pool will augment ongoing EMG activity, whereas an inhibitory input will suppress it (Fig. 40.1). Although this method is simple, it comes with certain limitations: (1) it can be used only on subjects able to perform a voluntary contraction for ∼ 1 to 2 minutes and, and (2) it suffers from a poor temporal resolution, and the changes in ongoing EMG following the early facilitation are difficult to interpret.1 Fig. 40.1 Common methods used to probe spinal circuitry in humans. From top to bottom: Y-shaped bars represent excitatory connections and small filled circles represent inhibitory connections. H-reflex: percutaneous electrical stimulation of a peripheral nerve, such as the median nerve innervating the flexor carpi radialis (FCR) muscle, results in an H-reflex on electromyography (EMG). The peak-to-peak amplitude of this response reflects the excitability of the myotatic arc (see Fig. 40.2 legend for more details). Ongoing rectified EMG: during a voluntary contraction of a target muscle (e.g., the biceps brachii muscle), the ongoing EMG activity is rectified and averaged (continuous line—unconditioned EMG). A conditioning electrical stimulation applied to the nerve supplying the antagonist muscle (e.g., the radial nerve innervating the triceps brachii muscle) results in a depression (arrow) in the EMG (dashed lines—conditioned EMG) due to reciprocal inhibition (see Fig. 40.3). Poststimulus time histogram (PSTH): changes in firing probability of a voluntary repetitively activated motor unit during a weak contraction of a target muscle (e.g., the FCR muscle) are determined by comparing the histogram of occurrence of motoneuronal (MN) discharges without homonymous nerve (e.g., median nerve) stimulation (filled histograms) and with homonymous nerve stimulation (opened histograms). The electrical stimulation induces a peak of occurrence of MN discharges due to the monosynaptic activation of a MN. Fig. 40.2 Monosynaptic reflexes and M wave. (A) Sketch of the pathway of the monosynaptic reflexes. Ia afferents from muscle spindle primary endings have monosynaptic connections to a motoneurons (MNs) innervating the muscle from which Ia afferents originate. The H-reflex is produced by electrical stimulation of Ia afferents that bypasses the muscle spindle, whereas the T-reflex stretches the muscle spindle and therefore also depends on the gamma drive. The M wave reflects the direct activation of the motor axon. Y-shaped bars represent excitatory connections. (B) Recruitment curve of H reflex and M wave in soleus muscle. A weak stimulation activating Ia afferents only (9 mA) results in the electromyogram (EMG) in an H-reflex (filled circles), which progressively augments in amplitude with the intensity of the stimulus. A moderate stimulus (15 mA) results in decreased H-reflex amplitude and an M wave (opened circles) appears because the threshold for motor axon activation is reached. A strong stimulus (30 mA) produces the maximal motor response (Mmax) and suppresses the H-reflex because of the collision between the reflex volley and the antidromic motor volley in the motor axons. Poststimulus time histograms (PSTHs) (Fig. 40.1) rely on the recording of a single isolated motor unit potential during a very weak voluntary contraction. In this technique, changes in firing probability of a voluntary repetitively activated motor unit are determined by constructing a histogram of occurrence of MN discharges, following repeated presentation of a conditioning stimulus. The main advantage of this method is that it allows for investigating single MNs and thus eliminates problems that are linked to the study of an MN pool (see earlier discussion). However, because this method is contingent upon the activation, by the subject, of an isolated motor unit at a rather stable frequency, it is challenging to use it in patients with motor control disorders. Changes in excitability of human MNs can also be investigated using F-waves, cortical stimulation, and coherence analysis between EMG/EMG or EMG/EEG signals (see Chapters 38 and 42). To summarize, each technique comes with its advantages and its limitations, given that these noninvasive methods are indirect and explore the excitability of spinal MNs through the “window” of EMG. To probe spinal circuits in healthy subjects, at rest and during various motor activities, the combined use of different methods allows for overcoming their specific drawbacks. In SCI patients, activation of MNs via peripheral inputs (H- or T-reflexes, F-waves) can be used regardless of the impairment of the motor command, whereas methods based on voluntary activation of MNs will be related to the latent motor command capabilities. In 1941, Renshaw2 showed in the cat that antidromic impulses flowing along motor axons inhibit homonymous and synergistic monosynaptic reflexes. This inhibition is due to interneurons (termed Renshaw cells), activated by axon recurrent collaterals, which project back to inhibit MNs (Fig. 40.3). Recurrent inhibition of a MN is the first feedback system described in the CNS and has been extensively studied in the cat.3 Renshaw cells have projections to (1) both α and γ MNs, even though finger and toe MNs have no recurrent inhibition, (2) Ia inhibitory interneurons, (3) other Renshaw cells, and (4) cells originating in the ventral spinocerebellar tract. Fig. 40.3 Diagram of reciprocal Ia inhibition at the ankle joint. Y-shaped bars represent excitatory connections and small filled circles represent inhibitory connections. Ia afferents from the soleus (Sol) muscle-spindle primary endings have monosynaptic connections to α motoneurons (MNs) and activate Ia interneurons (Ia INs), inhibiting MNs of the antagonist TA, and vice versa. Ia INs are inhibited by Renshaw cells and by “opposite” Ia INs (i.e., Sol-coupled Ia INs inhibit TA-coupled Ia INs and vice versa). The α (and γ) MNs and their corresponding Ia INs receive a parallel descending input from a higher center.
Basic Neurophysiological Approaches to Probing Spinal Circuits
 Methodological Overview
Methodological Overview
The Monosynaptic Reflex: H- and T-Reflexes
Ongoing Electromyographic Modulations
Poststimulus Time Histograms of the Discharge of Single Motor Units
 Recurrent Inhibition of a Motoneurons
Recurrent Inhibition of a Motoneurons
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basic Neurophysiological Approaches to Probing Spinal Circuits
